
User guide
Full face mask
User guide
English | Français
Español | Português
638035 AirFit F20 cover AMER Multi.indd 2 9/11/2016 11:06:49 AM

i
1
2
3 4
5
76
Fitting / Mise en place / Colocación / Colocação
638035 AirFit F20 cover AMER Multi.indd 1 9/11/2016 11:06:52 AM

ii
1 2
Adjustment / Ajustement / Ajuste / Ajuste
Removal / Retrait / Remoción / Remoção
638035 AirFit F20 cover AMER Multi.indd 2 9/11/2016 11:06:53 AM

iii
1 2
3
Disassembly / Démontage / Desensamblado /
Desmontagem
638035 AirFit F20 cover AMER Multi.indd 3 9/11/2016 11:06:54 AM

iv
1 2
Reassembly / Remontage / Reensamblado / Remontagem
638035 AirFit F20 cover AMER Multi.indd 4 9/11/2016 11:06:54 AM

v
Cleaning the vent / Nettoyage de l’orifice de ventilation
/ Limpieza del orificio de ventilación / Limpeza do
respiradouro
638035 AirFit F20 cover AMER Multi.indd 5 9/11/2016 11:06:54 AM

English 1
ENGLISH
Full face mask
Thank you for choosing the AirFit F20. This document provides the user
instructions for the AirFit F20 and AirFit F20 for Her masks referred to
collectively as AirFit F20 throughout this manual.
Using this guide
Please read the entire guide before use. When following instructions,
refer to the images at the front of the guide.
Intended use
The AirFit F20 is a non-invasive accessory used for channeling airflow
(with or without supplemental oxygen) to a patient from a positive airway
pressure (PAP) device such as a continuous positive airway pressure
(CPAP) or bilevel system.
The AirFit F20 is:
• to be used by patients weighing more than 66 lb (30 kg) for whom
positive airway pressure therapy has been prescribed
• intended for single-patient reuse in the home environment and multi-
patient reuse in the hospital/institutional environment.
WARNING
Magnets are used in the lower headgear straps and the frame of the
AirFit F20. Ensure the headgear and frame is kept at least 2" (50 mm)
away from any active medical implant (eg, pacemaker or
defibrillator) to avoid possible effects from localized magnetic fields.
The magnetic field strength is less than 400 mT.
Contraindications
Use of masks with magnetic components is contraindicated in patients
with the following pre-existing conditions:
• a metallic hemostatic clip implanted in your head to repair an
aneurysm
• metallic splinters in one or both eyes following a penetrating eye
injury.

2
GENERAL WARNINGS
• The mask must be used under qualified supervision for users who
are unable to remove the mask by themselves. The mask may not
be suitable for those predisposed to aspiration.
• The mask must be fitted with the supplied elbow (containing the
valve and vent assembly) to ensure safe and functional usage
unless otherwise specified. Do not use the mask if the valve or
vent assembly is damaged or missing.
• The elbow, valve and vent assembly have specific safety
functions. The mask should not be worn if the valve is damaged
as it will not be able to perform its safety function. The elbow
should be replaced if the valve is damaged, distorted or torn. The
vent holes and valve should be kept clear.
• The mask should only be used with CPAP or bilevel devices
recommended by a physician or respiratory therapist.
• Avoid connecting flexible PVC products (eg, PVC tubing) directly
to any part of the mask. Flexible PVC contains elements that can
be damaging to the materials of the mask, and may cause the
components to crack or break.
• The mask should not be used unless the device is turned on. Once
the mask is fitted, ensure the device is blowing air.
Explanation: CPAP and bilevel devices are intended to be used
with special masks (or connectors) which have venting to allow
continuous flow of air out of the mask. When the device is turned
on and functioning properly, new air from the device flushes the
exhaled air out through the mask holes. When the device is
turned off, the mask valve opens to atmosphere allowing fresh air
to be breathed. However, a higher level of exhaled air may be
rebreathed when the device is off. This applies to most full face
masks for use with CPAP and bilevel devices.
• Follow all precautions when using supplemental oxygen.
• Oxygen flow must be turned off when the CPAP or bilevel device
is not operating, so that unused oxygen does not accumulate
within the device enclosure and create a risk of fire.
• Oxygen supports combustion. Oxygen must not be used while
smoking or in the presence of an open flame. Only use oxygen in
well ventilated rooms.

English 3
• At a fixed rate of supplemental oxygen flow, the inhaled oxygen
concentration varies, depending on the pressure settings, patient
breathing pattern, mask, point of application and leak rate. This
warning applies to most types of CPAP or bilevel devices.
• The technical specifications of the mask are provided for your
clinician to check that they are compatible with the CPAP or
bilevel device. If used outside specification or if used with
incompatible devices, the seal and comfort of the mask may not
be effective, optimum therapy may not be achieved, and leak, or
variation in the rate of leak, may affect the CPAP or bilevel device
function.
• Discontinue using this mask if you have ANY adverse reaction to
the use of the mask, and consult your physician or sleep therapist.
• Using a mask may cause tooth, gum or jaw soreness or aggravate
an existing dental condition. If symptoms occur, consult your
physician or dentist.
• The F20 line of full face CPAP masks are not intended to be used
simultaneously with nebulizer medications that are in the air path
of the mask/tube.
• As with all masks, some rebreathing may occur at low CPAP
pressures.
• Refer to your CPAP or bilevel device manual for details on
settings and operational information.
• Remove all packaging before using the mask.
Using your mask
When using your mask with ResMed CPAP or bilevel devices that have
mask setting options, refer to the Technical specifications section in this
user guide for the correct setting.
For a full list of compatible devices for this mask, see the Mask/Device
Compatibility List on www.resmed.com/downloads/masks. If you do not
have internet access, please contact your ResMed representative.
Use a standard conical connector if pressure readings and/or
supplemental oxygen are required.

4
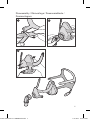
Fitting
1. Twist and pull both magnetic clips away from the frame.
2. Ensure that the ResMed logo on the headgear is facing outwards and
is upright. With both lower headgear straps released, hold the mask
against your face and pull the headgear over your head.
3. Bring the lower straps below your ears, and attach the magnetic clip
to the frame.
4. Undo the fastening tabs on the upper headgear straps. Pull the straps
evenly until the mask is stable and positioned as shown in the
illustration. Reattach the fastening tabs.
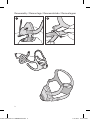
5. Undo the fastening tabs on the lower headgear straps. Pull the straps
evenly until the mask is stable and sits comfortably on the chin.
Reattach the fastening tabs.
6. Connect the device air tubing to the elbow. Attach the elbow to the
mask by pressing the side buttons and pushing the elbow into the
mask, ensuring it clicks in on both sides.
7. Your mask and headgear should be positioned as shown in the
illustration.
Adjustment
If necessary, slightly adjust the position of the mask for the most
comfortable fit. Ensure that the cushion is not creased and the headgear
is not twisted.
1. Turn on your device so that it is blowing air.
Adjustment tips:
With air pressure applied, pull the mask away from your face to
allow the cushion to inflate and reposition onto your face.
To resolve any leaks at the upper part of the mask, adjust the
upper headgear straps. For the lower part, adjust the lower
headgear straps.
Adjust only enough for a comfortable seal. Do not overtighten as
this may cause discomfort.
Removal
1. Twist and pull both magnetic clips away from the frame.
2. Pull the mask away from your face and back over your head.

English 5
Disassembly
If the mask is connected to your device, disconnect the device air tubing
from the elbow.
1. Undo the fastening tabs on the upper headgear straps. Pull the straps
out of the frame.
Tip: Keep the magnetic clips attached to the lower headgear straps to
easily distinguish the upper and lower straps when reassembling.
2. Remove the elbow from the mask by pressing the side buttons and
pulling the elbow away.
3. Hold the side of the frame between the upper and lower arms. Gently
pull the cushion away from the frame.
Reassembly
1. Attach the cushion to the frame by aligning the circular openings and
pushing together until retained.
2. With the ResMed logo on the headgear facing outside and upright,
thread the upper headgear straps into the frame slots from the inside.
Fold them over to secure.
Cleaning your mask at home
Handwash your mask and headgear by gently rubbing in warm
(approximately 86°F/30°C) water using mild liquid detergent. Rinse all
components well under running water and allow to air dry out of direct
sunlight.
WARNING
• As part of good hygiene, always follow cleaning instructions and
use a mild liquid detergent. Some cleaning products may damage
the mask, its parts and their function, or leave harmful residual
vapours that could be inhaled if not rinsed thoroughly.
• Regularly clean your mask and its components to maintain the
quality of your mask and to prevent the growth of germs that can
adversely affect your health.

6
CAUTION
Visible criteria for product inspection: If any visible deterioration of a
system component is apparent (cracking, discoloration, tears etc.),
the component should be discarded and replaced.
Daily/After each use:
1. Disassemble the mask according to the disassembly instructions.
2. Rinse the frame, elbow and cushion under running water. Clean with a
soft brush until dirt is removed.
3. Soak the components in warm water with a mild liquid detergent for
up to ten minutes.
4. Shake the components in the water.
5. Brush the moving parts of the elbow and around the vent holes.
6. Brush the areas of the frame where the arms connect, and inside and
outside the frame where the elbow connects.
7. Rinse the components under running water.
8. Leave the components to air dry out of direct sunlight. Make sure to
squeeze the arms of the frame to ensure that excess water is
removed.
Weekly:
1. Disassemble the mask. The magnets can remain attached to the
headgear during cleaning.
2. Handwash the headgear in warm water with mild liquid detergent.
3. Rinse the headgear under running water. Inspect to ensure the
headgear is clean and detergent free. Wash and rinse again, if
necessary.
4. Squeeze the headgear to remove excess water.
5. Leave the headgear to air dry out of direct sunlight.
Reprocessing the mask between patients
Only Sleep Lab Mask (SLM) variants of the mask are intended for multi-
patient re-use. When using between patients, these masks must be
reprocessed according to cleaning and disinfection instructions available
on ResMed.com/downloads/masks.

English 7
Troubleshooting
Problem/possible cause
Solution
Mask is uncomfortable.
Headgear straps are too tight. The cushion membrane is designed to inflate
against your face to provide a comfortable
seal with low headgear tension. Adjust straps
evenly. Ensure that the headgear straps are
not too tight and that the cushion is not
creased.
Mask could be the wrong size. Talk to your clinician to have your face size
checked against AirFit F20 fitting template.
Note that sizing across different masks is not
always the same.
Mask is too noisy.
Elbow incorrectly installed or incorrect
assembly of the mask system.
Remove the elbow from your mask, then
reassemble according to the instructions.
Check that the mask is correctly assembled
according to the instructions.
Vent is dirty. Use a soft bristle brush to clean the vent.
Mask leaks around the face.
Cushion membrane is creased. Mask is
incorrectly positioned or adjusted.
Refit your mask according to the instructions.
Ensure that you position the cushion correctly
on your face before pulling headgear over
your head. Do not slide the mask down your
face during fitting as this may lead to
creasing of the cushion.
Mask could be the wrong size. Talk to your clinician to have your face size
checked against fitting template. Note that
sizing across different masks is not always
the same.

8
Technical specifications
Pressure-flow curve
The mask contains passive venting to protect
against rebreathing. As a result of manufacturing
variations, the vent flow rate may vary.
Pressure
(cm H
2
O)
Flow
(L/min)
3
19
8
32
10
37
12
41
16
48
20
54
24
60
28
66
30
69
32
72
36
77
40
82
Dead space
information
Physical dead space is the empty volume of the mask to the end
of the swivel. Using the large cushions it is 240 mL.
Therapy pressure
3 to 40 cm H
2
O
Resistance with
Anti Asphyxia Valve
(AAV) closed to
atmosphere
Drop in pressure measured (nominal)
at 50 L/min: 0.2 cm H
2
O
at 100 L/min: 0.6 cm H
2
O
Inspiratory and
expiratory
resistance with Anti
Asphyxia Valve
(AAV) open to
atmosphere
Inspiration at 50 L/min:
0.6 cm H
2
O
Expiration at 50 L/min:
0.7 cm H
2
O

English 9
Anti Asphyxia Valve
(AAV) open-to-
atmosphere
pressure
≤3 cm H
2
O
Anti Asphyxia Valve
(AAV) closed-to-
atmosphere
pressure
≤3 cm H
2
O
Environmental
conditions
Operating temperature: 41°F to 104°F (5°C to 40°C)
Operating humidity: 15% to 95% non-condensing
Storage and transport temperature: -4°F to 140°F (-20°C to +60°C)
Storage and transport humidity: up to 95% non-condensing
Sound
DECLARED DUAL-NUMBER NOISE EMISSION VALUES in
accordance with ISO 4871. The A-weighted sound power level of
the mask is 30 dBA, with uncertainty of 3 dBA. The A-weighted
sound pressure level of the mask at a distance of 1 m is 23 dBA,
with uncertainty of 3 dBA.
Gross dimensions
Mask fully assembled with elbow assembly (no headgear)
6.1" (H) x 6.3" (W) x 5.8" (D)
(154 mm (H) x 159 mm (W) x 147 mm (D))
International
Commission on
Non-Ionizing
Radiation
Protection (ICNIRP)
Magnets used in this mask are within ICNIRP guidelines for
general public use.
Service life
The service life of the AirFit F20 mask system is dependent on the
intensity of usage, maintenance, and environmental conditions to
which the mask is used or stored. As this mask system and its
components are modular in nature, it is recommended that the
user maintain and inspect it on a regular basis, and replace the
mask system or any components if deemed necessary or
according to the ‘visual criteria for product inspection’ in the
‘Cleaning your mask at home’ section of this guide. Refer to the
‘Mask components’ section of this guide for information of how to
order replacement parts.

10
Mask setting options
For AirSense, AirCurve or S9: Select 'Full Face'.
For other devices: Select 'MIR FULL' (if available), otherwise
select 'FULL FACE' as the mask option.
Notes:
• This product is not made with PVC or phthalates such as DEHP, DBP
or BBP.
• This product is not made with natural rubber latex.
• The manufacturer reserves the right to change these specifications
without notice.
Storage
Ensure that the mask is thoroughly clean and dry before storing it for any
length of time. Store the mask in a dry place out of direct sunlight.
Disposal
This mask does not contain any hazardous substances and may be
disposed of with your normal household refuse.

English 11
Symbols
The following symbols may appear on your product or packaging:
Catalog number
Batch code
Humidity limitation
Temperature limitation
Fragile, handle with care
Not made with natural rubber
latex
Manufacturer
European Authorized
Representative
Keep away from rain
This way up
Full face mask
Device setting - Full Face
Size - small
Size - medium
Size - large
Indicates a Warning or Caution and alerts you to a possible injury or explains
special measures for the safe and effective use of the device
Caution, consult accompanying documents
Prescription only (In the US, Federal law restricts these devices to sale by or on
the order of a physician)

Limited warranty
ResMed Pty Ltd (ResMed) warrants that your ResMed mask system
(including mask frame, cushion, headgear and tubing) will be free from
defects in material and workmanship from the date of purchase for 90
days, or in the case of disposable masks and disposable mask
components for 7 days. This warranty is only available to the initial
consumer. It is not transferable. If the product fails under conditions of
normal use, ResMed will repair or replace, at its option, the defective
product or any of its components. This limited warranty does not cover: a)
any damage caused as a result of improper use, abuse, modification or
alteration of the product; b) repairs carried out by any service organization
that has not been expressly authorized by ResMed to perform such
repairs; and c) any damage or contamination due to cigarette, pipe, cigar
or other smoke. Warranty is void on product sold, or resold, outside the
region of original purchase.
Warranty claims on defective product must be made by the initial
consumer at the point of purchase.
This warranty replaces all other expressed or implied warranties, including
any implied warranty of merchantability or fitness for a particular purpose.
Some regions or states do not allow limitations on how long an implied
warranty lasts, so the above limitation may not apply to you.
ResMed will not be responsible for any incidental or consequential
damages claimed to have resulted from the sale, installation or use of any
ResMed product. Some regions or states do not allow the exclusion or
limitation of incidental or consequential damages, so the above limitation
may not apply to you. This warranty gives you specific legal rights, and
you may also have other rights which vary from region to region. For
further information on your warranty rights, contact your local ResMed
dealer or ResMed office.
12

Français 1
FRANÇAIS
Masque facial
Merci d'avoir choisi l'AirFit F20. Ce document indique comment utiliser
les masques AirFit F20 et AirFit F20 for Her, désignés par AirFit F20 dans
le présent manuel.
Utilisation de ce guide
Veuillez lire le guide entièrement avant d’utiliser ce masque. Les
instructions doivent être lues en consultant les illustrations au début de
ce manuel.
Usage prévu
L'appareil AirFit F20 est un accessoire non invasif utilisé pour acheminer
au patient le débit d'air (avec ou sans adjonction d'oxygène) produit par
un appareil à pression positive tel qu'un appareil de PPC ou à deux
niveaux de pression.
L'AirFit F20 est prévu pour :
• une utilisation par des patients pesant plus de 66 lb (30 kg) à qui un
traitement par pression positive a été prescrit;
• un usage multiple par un seul patient à domicile ou un usage multiple
par plusieurs patients en milieu médical.
AVERTISSEMENT
Des aimants sont utilisés dans les sangles inférieures du harnais et
dans l'entourage rigide de l'AirFit F20. Vérifiez que le harnais et
l'entourage rigide soient maintenus à une distance minimale de
50 mm (2 pouces) de tout implant médical actif (p. ex. un pacemaker
ou un défribillateur) pour éviter les effets possibles des champs
magnétiques localisés. La force du champ magnétique est inférieure
à 400 mT.

2
Contre-indications
L'utilisation des masques avec des composants magnétiques est contre-
indiqué chez les patients présentant les caractéristiques pré-existantes
suivantes :
• dispositif hémostatique métallique implanté dans le crâne suite à un
anévrisme;
• éclats métalliques dans un œil ou dans les deux yeux suite à un
traumatisme oculaire pénétrant.
AVERTISSEMENTS D’ORDRE GÉNÉRAL
• Le masque doit être utilisé sous le contrôle d'une personne
qualifiée si le patient n'est pas en mesure de l'enlever de lui-
même. Le masque peut ne pas convenir aux patients sujets aux
aspirations trachéo-bronchiques.
• Le masque doit être équipé du coude qui l'accompagne (et
contenant la valve et l'orifice de ventilation) afin d'assurer une
utilisation sûre et pratique, sauf si autrement spécifié. Ne pas
utiliser le masque si la valve ou l'orifice de ventilation sont
endommagés ou manquants.
• L’ensemble coude, valve et orifice de ventilation possède des
caractéristiques de sécurité spécifiques. Le masque ne doit pas
être porté si la valve est endommagée, auquel cas elle n'est pas
en mesure de remplir sa fonction de sécurité. Le coude doit être
remplacé si la valve est endommagée, déformée ou déchirée. Les
orifices de ventilation et la valve ne doivent jamais être obstrués.
• Le masque doit être utilisé uniquement avec les appareils de PPC
ou à deux niveaux de pression recommandés par un médecin ou
un kinésithérapeute respiratoire.
• Éviter de raccorder des produits en PVC souple (par ex. un circuit
en PVC) directement aux composants de ce masque. Le PVC
souple contient des éléments qui peuvent endommager les
matériaux du masque et entraîner leur fissuration ou leur rupture.
• Le masque ne doit être porté que si l'appareil est sous tension.
Une fois le masque en place, s'assurer que l'appareil produit un
débit d'air.
Explication : Les appareils de PPC et à deux niveaux de pression
sont conçus pour une utilisation avec des masques (ou raccords)
spéciaux possédant des orifices de ventilation qui permettent
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
ResMed AirFit F20 Manuel utilisateur
- Taper
- Manuel utilisateur
- Ce manuel convient également à
dans d''autres langues
- English: ResMed AirFit F20 User manual
- español: ResMed AirFit F20 Manual de usuario
- português: ResMed AirFit F20 Manual do usuário
Documents connexes
-
ResMed AirTouch F20 Mode d'emploi
-
ResMed AirFit F20 Mode d'emploi
-
ResMed AirTouch F20 Mode d'emploi
-
ResMed AirFit F10 Full Face Mask Manuel utilisateur
-
ResMed AirFit F10 for Her Mode d'emploi
-
ResMed AirFit F10 for Her Mode d'emploi
-
ResMed AirFit F10 for Her Manuel utilisateur
-
ResMed AirTouch F20 Manuel utilisateur
-
ResMed AirTouch F20 Mode d'emploi
-
ResMed AirTouch F20 Manuel utilisateur



























































