With a CryoGrid located in the left CryoBath, and an additional CryoGrid or CryoTransporter located in the
right CryoBath (Figure B), refer to the lower screen.
If you have additional CryoBeacons for this procedure, repeat Step 2 and Step 3.
Wait for the RFID Readerboard to map the
CryoBeacons in the CryoGrid and CryoTransporter.
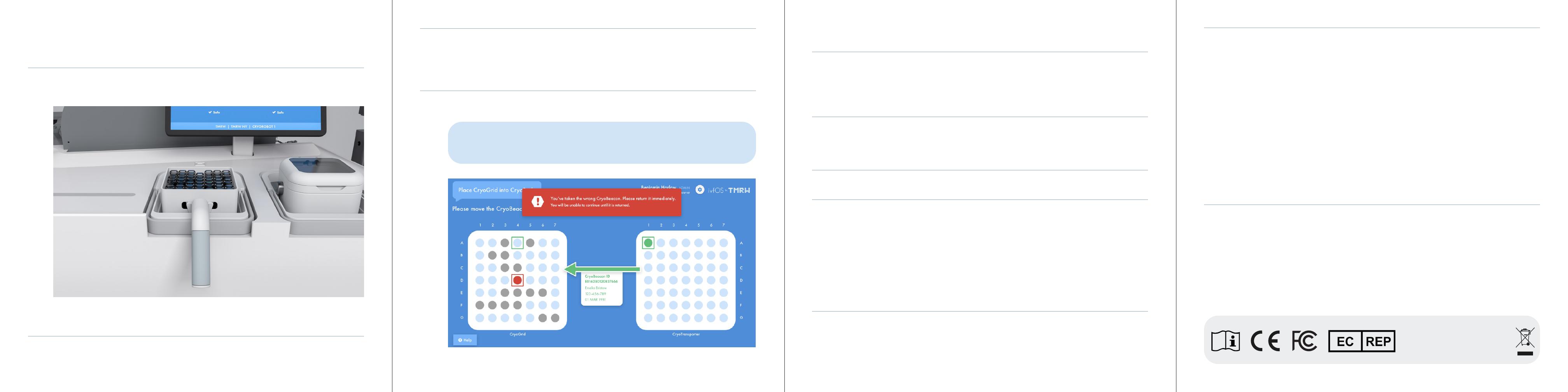
Following the arrow on the screen, move the CryoBeacon highlighted in green to the empty location
highlighted in green. After a CryoBeacon has been moved, the RFID Readerboard updates the screen to
reflect the movement of the CryoBeacon.
01
04
02
03
INSTRUCTIONS FOR USE
Medical Product Service GmbH (MPS)
Borngasse 20
35619 Braunfels, Germany
Note: For specimen vitrification, you can use the CryoTransporter or your preferred cryogenic container. If you
choose to use the CryoTransporter for vitrification, the angled slot in the front allows for easy and safe loading of
your cryodevice into the CryoBeacon. The CryoTransporter provides sufficient LN2 depth to plunge cryodevices
during vitrification.
NOTICE
1. FCC CFR 47 15.19
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) this device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that may cause undesired operation.
2. FCC CFR 47 15.21
Caution: any changes or modifications to this device not expressly approved by TMRW Life Sciences, Inc. could
void the user’s authority to operate the equipment.
3. FCC CFR 47 15.105 INFORMATION TO THE USER
4. (a) For a Class A digital device or peripheral, the instructions furnished the user shall include the following or
similar statement, placed in a prominent location in the text of the manual:
Note: This equipment has been tested and found to comply with the limits for a Class A digital device, pursuant to
part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference
when the equipment is operated in a commercial environment. This equipment generates, uses, and can radiate
radio frequency energy and, if not installed and used in accordance with the instruction manual, may cause harmful
interference to radio communications. Operation of this equipment in a residential area is likely to cause harmful
interference in which case the user will be required to correct the interference at his own expense.
5. FCC RADIATION EXPOSURE STATEMENT:
This device complies with FCC RF radiation exposure limits set forth for an uncontrolled environment. This transmitter
must not be co-located or operating in conjunction with any other antenna or transmitter. The equipment should be
installed and operated with minimum distance of 20cm between the radiator and your body.
6. ISED STATEMENTS
This device contains license-exempt transmitter(s)/receiver(s) that comply with Innovation, Science and Economic
Development Canada’s licence exempt RSS(s). Operation is subject to the following two conditions:
(1) This device may not cause interference.
(2) This device must accept any interference, including interference that may cause undesired operation of the device.
Ce dispositif contient les émetteurs/récepteurs autoriser-exempts qui sont conformes au permis RSS exempt du Canada
d’innovation, de la Science et de développement économique. L’opération est sujette aux deux conditions suivantes:
(1) Ce dispositif peut ne pas causer l’interférence.
(2) Ce dispositif doit accepter n’importe quelle interférence, y compris l’interférence qui peut causer le
fonctionnement peu désiré du dispositif.
CAN ICES-3 (A) / NMB-3 (A)
This Class A digital apparatus complies with Canadian ICES-003.
Cet appareil numérique de classe A est conforme à la norme canadienne ICES-003.
7. RADIATION EXPOSURE STATEMENT: ISED
This equipment complies with ISED radiation exposure limits set forth for an uncontrolled environment. This equipment
should be installed and operated with greater than 20cm between the radiator and your body.
Déclaration d’exposition aux radiations:
Cet équipement est conforme aux limites d’exposition aux rayonnements ISED établies pour un environnement non
contrôlé. Cet équipement doit être installé et utilisé à plus de 20 cm entre le radiateur et votre corps.
Innovation, Science and Economic Development Canada ICES 003 Compliance Label: CAN ICES-3 (A)/NMB-3(A)
Figure B: CryoGrid and CryoTransporter in CryoBaths
Figure C: Lower Screen - CryoBeacon Error


