
Read carefully before using
this device
Model No. KTR-2302
*pain relief is temporary
User Manual
EN
freedom from period pain

2
Table of Contents
The serious stuff
Indications for Use and Intended User 3
Who should NOT use Ellune – Contraindications 3
Warnings and Cautions 4
Contents of your Ellune pack 6
Device overview and diagrams 8
Everything you need to know about using Ellune
Before using for the first time 9
Device set-up 9
Positioning the electrodes 10
Applying the electrodes 10
Connecting the electrodes to Ellune 10
Using Ellune 10
Lock function 11
Connection error 11
Finishing a session 11
Taking care of your Ellune
Replacing the gel pads 12
Charging your Ellune 12
Cleaning and Maintenance 12
Frequently Asked Questions 13
Troubleshooting 14
Technical Details
Device Specification 15
Glossary of Graphic symbols 16
Safety and compliance standards 17
EMC & Electrical standards/requirements 17
Electromagnetic emissions tables 19
Device lifespan 22
Manufacturer and EC rep details 22

3
EN
The serious stuff
Indications for use
Ellune is a TENS device for the temporary relief of menstrual pain and
discomfort for primary dysmenorrhea, when the pain associated with
menstruation is not caused by any underlying gynaecological disorder
Intended user
Ellune should be used only by those aged 16 and above, that are still having
menstrual periods.
Who should NOT use Ellune?
CONTRAINDICATIONS
DO NOT USE:
eIf you have suspected or confirmed epilepsy or heart disease,
malignant tumour, serious cerebrovascular disease or other acute
disease, unless specialist medical authorisation has first
been obtained.
eIf you are fitted with a pacemaker or other active implanted electrical
device. Such use could cause electric shock, burns, electrical
interference, or death.
eIn case of epilepsy or suspicion of epilepsy because electro
stimulation could promote the occurrence of epileptic seizures.
eThis device is not intended for and should not be used by those who
are pregnant, in labour, or breastfeeding.
eEllune should not be used while fertility problems are being evaluated,
diagnosed, or treated.
eDo not use in areas with staples, implants or any metal parts in order
to avoid the risk of burns or pain.
eOn children or people with no ability to express their
own consciousness.
eIf you have been diagnosed with or have any reason to believe that you
may be suffering from, Deep Vein Thrombosis (“DVT”).
eIf suffering from haemorrhage after acute trauma or fractures, or if
recovering from surgery.

4
ADVERSE REACTIONS
eUse of the device may cause irritation to the skin beneath
the electrodes.
eBurns beneath the electrodes have also been reported in
the literature.
eApplication of electrodes near the thorax may increase the risk of
cardiac fibrillation.
eAllergic reactions to the gel adhesive may be possible.
eLong-term stimulation of the same area may cause skin irritation.
WARNINGS
eDo not place electrodes over the neck because this could cause severe
muscle spasms resulting in closure of the airway, difficulty breathing,
or adverse effects on heart rhythm or blood pressure.
eDo not place electrodes across the chest because the introduction of
electrical current into the chest may cause rhythm disturbances to the
heart which could be lethal.
eDo not place electrodes over open wounds, rashes, or over swollen,
red, infected, or inflamed areas or skin eruptions (e.g., phlebitis,
thrombophlebitis, and varicose veins).
eDo not place electrodes over, or in proximity to, cancerous lesions.
eDo not place electrodes on the genitals.
eElectrodes should be applied only to normal, intact, clean and
healthy skin.
eIncorrect application of electrodes could result in discomfort or
burning of the skin.
eDo not share electrodes with other persons because of the risks of
adverse skin reactions and disease transmission.
eAs Ellune is a TENS device, it is intended for symptomatic treatment
and has no curative effect.
eElectronic devices (such as an ECG and others) may not operate
properly when TENS stimulation is in progress.
CAUTION:
eTurn Ellune off before applying or removing the electrodes.
eTENS is not recommended for patients with heart disease who have
not received a medical evaluation of possible adverse effects.
eTENS should not be used to alleviate an undiagnosed pain syndrome
until the etiology has been well established.
eEllune should not be used for ovulation pain (mid-cycle pain).
eEllune should not be used while fertility problems are being evaluated,
diagnosed, or treated.

5
EN
eEllune should not be used during pregnancy, labour or breastfeeding.
eBatteries are rechargeable; batteries should not be replaced by
unauthorised personnel.
eTo avoid cross-contamination, electrodes are intended to be used by
one person only.
eTo avoid potential contamination, use the electrodes only on intact skin
for no longer than 10 hours at a time.
eKeep Ellune dry. Do not expose the device to a wet environment and do
not use when bathing or showering.
CAUTIONS
eUse caution while using Ellune in tandem with any monitoring
equipment with body-worn electrode pads. Ellune may interfere with
the signals being monitored.
eStrong electromagnetic fields may affect the correct operation of
this device. If unusual phenomena are observed, move away from
electromagnetic fields.
eUse caution following recent surgical procedures. Electrical
stimulation may disrupt the healing process.
eSince the effects of stimulation of the brain are unknown, stimulation
electrodes should not be placed on opposite sides of the head.
eThe long-term effects of cutaneous electrodes for electrical
stimulation on the human body are unknown.
eKeep electrodes out of the reach of children.
eUse caution if electrodes are applied over areas of skin that lack
normal sensation.
eReplace self-adhesive electrodes if they no longer stick firmly to
the skin.
eDo not charge the device while using it.
eDo not operate the device when electrodes are not connected. Make
sure the device is off before removing or connecting the electrodes.
eUse caution, if operating machinery or driving, to ensure that electrical
stimulation or involuntary muscle contraction is not dangerous.
eDo not use this device while you sleep to avoid the risk of pain,
inflammation, or burns due to excessive intensity adjustment that you
would not be able to control for lack of alertness.
Do not place electrodes:
eOn broken skin.
eOn skin which does not have normal sensation. If the skin is numb, too
great a strength may be used in the Ellune and this could result in
skin inflammation.

6
eOn the front of the neck. Doing so could cause the airway to close,
affecting breathing, or causing a sudden drop in blood pressure
(vasovagal response).
eOver the eyes. Doing so may affect eyesight or cause headaches.
eAcross the front of the head. The effect on patients who have had
strokes or seizures is unknown.
eOver, or in proximity to, cancerous lesions.
Do not:
eIgnore any allergic reaction to the electrodes. If skin irritation
develops, stop using the device.
eImmerse your device unit in water or place it close to excessive
heat – this may cause the device to cease operating correctly.
eAttempt to open up the device - there are no serviceable components
and there is a risk of electric shock.
eUse this device with electrodes other than those recommended by the
manufacturer – performance and safety may be compromised.
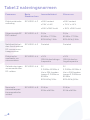
Contents of your Ellune pack
Silicone cover
This fits over your Ellune. Different
colours are available allowing you
to customise your device.
Charging cable
Lets you connect your Ellune to a
USB point to charge the battery
Ellune device
The main unit that lets you control
the therapy.

7
EN
2x electrode pads
These pads are placed on your skin,
at the point of pain, where they
deliver Ellune’s therapy. They are
connected by cable to the
Ellune unit.
Connecting cable
Connects the electrode pads to the
Ellune device.
Carry bag
Keep Ellune and its accessories in this bag
for protection and easy transport.
www.paingone.com
6x gel pads
These replaceable gel pads
help the electrode pads
adhere to your skin.
If any part is missing, or to purchase accessories for your
Ellune, contact your local distributor or visit
User manual
The manual you have in your hands right now!
Quick start guide

8
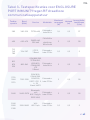
Device overview and diagrams
Device front view
Reverse view Electrode pads
Side view
Display screen
ePower On/Off button
eIncrease Intensity button
eDecrease Intensity button
eLock function button
eDisplay screen
eConnecting Cable
eElectrode Pad
eAttaching clip – Allows you to
attach Ellune to your waistband
eUSB-C Connection point
eBattery level
eConnection
error indicator
eLock function
indicator eIntensity level

9
EN
Everything you need to
know about using Ellune
Before using for the first time
We recommend charging your Ellune before using
for the first time. This will give your device the best
possible start and ensure it performs optimally.
1. Connect the charging cable to Ellune
2. Connect the cable to a USB charger
3. We recommend charging for 2 hours this first time.
All future charges take approximately 1 hour.
Device Set-up
1. Take the electrode pads.
2. Attach the first pad to the
connecting cable. The pads
connect via metal press studs.
Press until the connection snaps
firmly into place.
3. Repeat this action with the
second pad.
4. Take a gel pad. Remove a plastic
liner from one side of the pad (be
sure to retain liners for later)
5. Apply the gel pad to the reverse
(the black side) of on
electrode pad.
6. Repeat the above with the
second gel pad, applying it to the
second electrode pad.
connect
Scan the QR
code to watch a
‘how-to’ video
6
1
2
3
4
5

10
Positioning the electrode pads
In the next step we will apply the pads
to the required area. You can apply them
on the abdomen or the lower back,
depending on where you have the
most pain.
Aim to position the pads on either side
of the painful area, at the same height,
and at a distance of 5-10cm apart from
each other.
Using Ellune
1. Press the power button to
activate Ellune.
2. Set the intensity to a level you
find comfortable. Use the “+”
button to increase the intensity.
You can tap it to increase by just
one level. Or hold the button
down to change levels rapidly.
If the intensity becomes too
strong, press the “-“ button to
reduce intensity.
3. The screen displays your
chosen intensity level. There
are 35 levels in total.
+
+
+++
Connecting the pads to Ellune
1. Now, take the connecting cable and connect this to your Ellune device.
Applying the electrode pads
1. Ensure that your desired area of application is clean and dry.
2. Remove the remaining plastic liners from the gel pads.
3. Apply the pads as required on the abdomen or lower back.

11
EN
Lock function
To avoid accidentally changing the intensity
or turning the device off during use, press the
‘lock’ button to activate lock mode. This will
disable all the other buttons. Simply press the
‘lock’ button once more to exit lock mode.
Connection error
If Ellune detects that there is a connection
error, it will pause therapy and display the connection
error symbol on the display screen.
To resolve a connection error, check that:
- The gel pads are applied firmly to your skin
- The connecting cable is correctly attached to the pads and Ellune device
Once connection is re-established, Ellune will automatically resume therapy
and return to the intensity level that was previously selected.
Over time, the connecting cable and/or electrode pads may deteriorate
from daily wear and tear. These parts can be replaced. Contact your local
distributor or visit www.paingone.com for replacements.
Finishing a session
1. To stop the Ellune therapy, simply press the power button.
2. Carefully remove the pads from your skin.
3. Place a plastic liner back over each gel pad. This will keep them in
optimum condition for their next use.
4. You can store Ellune and its accessories in the carry bag supplied.
4. Once you are comfortable, you can attach Ellune to your waistband
using the clip on the reverse of the device.
5. Let Ellune do it’s work while you get on with the things you love. The
device will run until you press the power button or the battery
is exhausted.

12
Taking Care of Your Ellune
Replacing the gel pads
For optimum effectiveness and hygiene, we recommend using one set of
gel pads per menstrual cycle.
At the start of your menstrual cycle, remove and dispose of the used gel
pads and replace with brand new pads.
Ensure your device is switched off when changing the gel pads.
Charging your Ellune
When Ellune’s battery is getting low, the
device will beep and the battery symbol on the
display will flash. This indicates that it is time to
recharge your device.
Take the charging cable supplied. Connect this into your Ellune device, and
connect the other end to a USB charger.
The battery symbol on the screen will show that the device is charging .
Once fully charged, the battery symbol will display 4 solid bars.
Ellune is compatible with fast-chargers. It takes approximately 1 hour to
fully charge the battery.
Cleaning and maintenance
Switch off Ellune before cleaning. Use a disinfectant or anti-
microbiological wipe.
Wipe all external surfaces of the device control unit and all cables.
Care should be taken not to clean the cable connectors.

13
EN
Frequently Asked Questions
How long can I use Ellune for?
Ellune can be used as long as you are experiencing period pain.
The electrode pads should be removed and the site of application adjusted
at least every 10 hours. Regularly changing the site of application will
reduce the risk of skin irritation.
What is the right intensity level for me?
The right intensity level is one that feels comfortable for you and where it
begins to match the intensity of your pain.
How does Ellune work?
Ellune works by sending high-frequency electrical signals that are both
continuous and mild to block out the pain signals being delivered to the
brain. This is sometimes known as the “gate” control effect because it
reduces transmission of pain. When these pain signals are halted, pain
is not felt by the reactive area and the patient gets relief. Electrotherapy
also helps activate the natural pain control response, releasing beta
endorphins that ease the pain felt by the patient.
Can I use Ellune in combination with medication?
Yes, you can use Ellune in combination with medication you may be taking
Where can I obtain replacement parts and accessories?
Visit www.paingone.com or contact your local distributor
Can I place one pad on my abdomen and one on my back?
The maximum distance apart the electrode pads can be placed is 10cm.
To apply Ellune to the abdomen and the back at the same time requires 2x
Ellune devices.

14
Issue Suspected cause Suggested action
No stimulation
from pads
Low battery or
connection error.
Fully charge Ellune's battery.
Check that the connection cable
is properly connected to the
electrode pads and to your Ellune
device, and that the cable is not
damaged.
No stimulation
from electrode
pads. Screen
displays
Connection error.
The electrode
pads may have
come loose from
the skin. Or the
connecting cable
may have detached
or be damaged.
Firmly press the electrode pads
against your skin. Check that
the connection cable is properly
connected to the pads and to your
Ellune device. Replace cable if
there are signs of damage.
Stimulation
feels weak
Poor connection or
worn gel pads
Firmly press the electrode pads
against your skin. If this does not
improve stimulation, apply brand
new gel pads to the
electrode pads.
Device beeps Low battery.
The beep sound
indicates that
battery is running
out
Fully charge Ellune's battery
Nothing displayed
on screen
Exhausted battery Fully charge Ellune's battery
Gel does not
adhere to the skin
Gel pads have
become overused
Replace the gel pads
Stimulation is felt
more strongly
through one
electrode pad
Poor positioning of
electrode pads
Ensure the electrode pads are
positioned on either side of your
pain and at the same height on
your body
Buttons are
not responsive
Lock function may
be active
Press the lock button once to
reactivate the other buttons
Troubleshooting

15
EN
Technical Details
Device specification
Model No: KTR-2302
Output: 0-45V
Intensity: 35 levels
Frequency: 100Hz
Pulse width: 120µ
Weight: 44g
Dimensions: 75×50×19.8mm
Power consumption: About 0.2W
Power source: 3.7V DC 500mAh
Power supply: Type-C USB 5V/9V DC
Input Power: 1.5W
Battery life cycles: Under normal use, the battery life is ≥500 recharge
cycles, about 2-3 years of use (30 minutes per day).
a. Environment for operation
Temperature: +5°C~+40°C
Humidity: 0%~80%
Barometric pressure: 86kpa~106kpa
a. Environment for storage:
Temperature: -25°C~+55°C;
Humidity: 0-93%RH
Barometric pressure: 50kpa~106kpa
a. Environment for transport
Temperature: -25°C~+55°C;
Humidity: 0-93%RH
Barometric pressure: 50kpa~106kpa

16
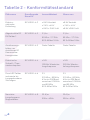
Glossary of Graphic Symbols
Symbol Explanation
Production Batch
Product catalogue reference code
Manufacturer (Directive 93/42/EEC amended by
Directive 2007/47/EC)
DATE OF MANUFACTURE. This symbol shall be
accompanied by a date to indicate the date of
manufacture
Caution
Applied part of type BF
European Authorized Representative
Do not dispose in household waste. Separate
collection for waste electric and electronic
equipment (WEEE) is required
CE marking with the Registration Number of the
Notified Body. This denotes compliance with European
Directive 93/42/EEC concerning medical devices.
Level of protection against the insertion of solid
bodies of size/diameter ≥ 12 mm and liquids in
the presence of dripping water when tilted at 15°
compared with products.
Refer to instruction manual/ booklet

17
EN
Safety and compliance standards
The product conforms to the following standards and laws:
1. IEC 60601-1:2005+A1:2012 Medical electrical equipment-Part 1: General
requirements for basic safety and essential performance 2. IEC 60601-
1-11:2015 Medical Electrical Equipment - Part 1-11: General Requirements
For Basic Safety And Essential Performance - Collateral Standard:
Requirements For Medical Electrical Equipment And Medical Electrical
Systems Used In The Home Healthcare Environment
3. IEC 60601-2-10: 2012+A1:2016 Medical electrical equipment - Part 2-10:
Particular requirements for the safety of nerve and muscle stimulators
4. IEC 60601-1-2:2014 Medical Electrical Equipment - Part 1-2: General
Requirements For Basic Safety And Essential Performance - Collateral
Standard: Electromagnetic Disturbances - Requirements And Tests
EMC & electrical standards/requirements
IEC 60601-1-2:2014 Medical Electrical Equipment - Part 1-2: General
Requirements For Basic Safety And Essential Performance - Collateral
Standard: Electromagnetic Disturbances - Requirements And Tests
The equipment is intended for use in the electromagnetic environment
specified below.
The customer or the user of the EQUIPMENT should assure that it is used in
such an environment.
This Transcutaneous Electrical Nerve Stimulators is suitable for use in a
professional health care environment, not including areas where there are
sensitive equipment or sources of intense electromagnetic disturbances,
such as the RF shielded room of a magnetic resonance imaging system,
in operating rooms near active AF surgical equipment, electrophysiology
laboratories, armored rooms or areas where short wave therapy
equipment is used.

18
eDo not use the system around strong electric fields, electromagnetic
fields (e.g. MRI scan room) and mobile wireless communication
devices. Using the device in an improper environment may cause
malfunction or damage.
eThe compliance with EMC and EMI regulation cannot be guaranteed by
the use of modified cables or those which do not comply with the same
standards under which the equipment was validated.
eThe system must not be used adjacent to or supported by other
equipment.
eThe recommendations of this manual must be followed.
eDo not use accessories, transducers, internal parts of components
and other cables other than those previously specified by the
manufacturer. This may result in increased emission or decreased
electromagnetic immunity and result in improper operation.
ePortable RF communications equipment (including peripherals
such as antenna cables and external antennas) should by used no
closer than 30cm to any part of the ultrasound system, including
cables specified by the manufacturer. Otherwise, degradation of the
performance of this equipment could result.
eTo maintain basic safety in relation to electromagnetic disturbances
during the expected service life, always use the system in the
specified electromagnetic environment and follow the maintenance
recommendation described in this manual.
The following tables provide information on compliance of the equipment
according to the standard EN 60601-1- 2:2015.

19
EN
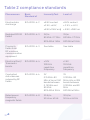
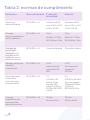
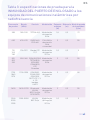
Electromagnetic emissions table
Table 1 compliance class
Emissions Test Compliance Electromagnetic Environment
and Guidance
RF emissions
CISPR 11
Group 1 The equipment uses RF
energy only for its internal
function. Therefore, its RF
emissions are very low
and are not likely to cause
any interference in nearby
electronic equipment.
RF emissions
CISPR 11
Class B The equipment is suitable for
use in all establishments,
including domestic
establishments and those
directly connected to the
public low-voltage power
supply network that supplies
buildings used for
domestic purposes.
Harmonic
emissions
IEC 61000-3-2
Class A
Voltage
fluctuations/
flicker emissions
IEC 61000-3-3
Complies

20
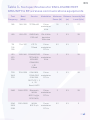
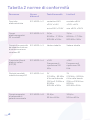
Table 2 compliance standards
Phenomenon Basic
Standard of
Immunity Test Level of
Electrostatic
discharge
IEC 61000-4-2 ±8 KV contact
±2 KV, ±4 KV,
±8 KV,±15KV air§
±8 KV contact
± 2 K V, ± 4 K V,
± 8 KV, ±15KV air
Radiated RF EM
fields1
IEC 61000-4-3 3V/m
80 MHz-2.7 GHz
80% AM at 1 KHz
3V/m
80 MHz-2.7 GHz
80% AM at 1 KHz
Proximity
fields from
RF wireless
communication
equipment
IEC 61000-4-3 See table See table
Electrical Fast/
Transients
bursts
IEC 61000-4-4 ±1 KV
100 KHz
repetition
frequency
±1 KV
100 KHz
repetition
frequency
Conducted
disturbances
induced by RF
fields.
IEC 61000-4-6 3V
0.15 MHz-80
MHz 6 Vm in ISM
bands between
0 .15 MHz and
80 MHz
80% AM at 1KHz
3V
0.15 MHz-80
MHz 6 Vm in ISM
bands between
0.15 MHz and 80
MHz
80% AM at 1KHz
Rated power
frequency
magnetic fields
IEC 61000-4-8 30 A/m
50 Hz or 60 Hz
30 A/m
50 Hz or 60 Hz
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
-
 65
65
-
 66
66
-
 67
67
-
 68
68
-
 69
69
-
 70
70
-
 71
71
-
 72
72
-
 73
73
-
 74
74
-
 75
75
-
 76
76
-
 77
77
-
 78
78
-
 79
79
-
 80
80
-
 81
81
-
 82
82
-
 83
83
-
 84
84
-
 85
85
-
 86
86
-
 87
87
-
 88
88
-
 89
89
-
 90
90
-
 91
91
-
 92
92
-
 93
93
-
 94
94
-
 95
95
-
 96
96
-
 97
97
-
 98
98
-
 99
99
-
 100
100
-
 101
101
-
 102
102
-
 103
103
-
 104
104
-
 105
105
-
 106
106
-
 107
107
-
 108
108
-
 109
109
-
 110
110
-
 111
111
-
 112
112
-
 113
113
-
 114
114
-
 115
115
-
 116
116
-
 117
117
-
 118
118
-
 119
119
-
 120
120
-
 121
121
-
 122
122
-
 123
123
-
 124
124
-
 125
125
-
 126
126
-
 127
127
-
 128
128
-
 129
129
-
 130
130
-
 131
131
-
 132
132
dans d''autres langues
- italiano: PAINGONE 15229690 Istruzioni per l'uso
- español: PAINGONE 15229690 Instrucciones de operación
- Deutsch: PAINGONE 15229690 Bedienungsanleitung
- Nederlands: PAINGONE 15229690 Handleiding
Autres documents
-
Omron PM3032 Manuel utilisateur
-
Drive Medical Padded Swivel Seat Cushion Manuel utilisateur
-
 NURSAL PL-029U Mode d'emploi
NURSAL PL-029U Mode d'emploi
-
Beurer EM 44 Le manuel du propriétaire
-
Terraillon Easy Care Le manuel du propriétaire
-
Terraillon Trio Care Le manuel du propriétaire
-
Terraillon Trio Care Mode d'emploi
-
 TensCare UNICARE Manuel utilisateur
TensCare UNICARE Manuel utilisateur
-
Beurer S02961 Mode d'emploi
-
 TensCare ALIVIA Manuel utilisateur
TensCare ALIVIA Manuel utilisateur






































































































































