
1
BACK AND KNEE PAIN RELIEF

2
EN

3
TABLE OF CONTENTS
Introduction .............................................................................................................................................................................................4
Indications for Use ................................................................................................................................................................................4
Safety Warning ...................................................................................................................................................................................... 5
Contraindications .................................................................................................................................................................................. 5
Warnings .................................................................................................................................................................................................. 5
Precautions .............................................................................................................................................................................................6
Adverse Reactions ................................................................................................................................................................................8
Symbol and Title ...................................................................................................................................................................................8
Environmental Condition for Transport and Storage .............................................................................................................. 9
Electromagnetic Compatibility ...................................................................................................................................................... 10
How the Device Works...................................................................................................................................................................... 14
Device Description ..............................................................................................................................................................................15
Operating Instruction ........................................................................................................................................................................ 16
Performance Specifications............................................................................................................................................................ 21
Cleaning and Maintenance ............................................................................................................................................................. 21
Trouble Shooting ................................................................................................................................................................................ 22
Contact Information .......................................................................................................................................................................... 23

4
INTRODUCTION
Compex Tens / Heat delivers electric pulses and heat to the user’s body areas such as knee and back
through the conductive silver pads. The portable and compact device has multiple modes of different
pulse frequencies, covering Transcutaneous Electrical Nerve Stimulation (TENS) and Electrical Muscle
Stimulation (EMS). It includes operating elements of ON/OFF button, intensity increase button, intensity
decrease button, mode button, timer button, and heat/temperature button, and can be attached and
detached to a wrap with conductive silver pads through magnetic connectors.
INDICATIONS FOR USE
To be used for temporary relief of pain associated with sore and aching muscles due to strain from exercise
or normal household and work activities.
It is also intended for symptomatic relief and management of chronic, intractable pain and relief of pain
associated with arthritis.
To stimulate healthy muscles in order to improve and facilitate muscle performance. To be used for the
improvement of muscle tone and firmness, and for strengthening muscles. Not intended for use in any
therapy or for the treatment of any medical conditions or diseases.
It is also intended to temporarily increase local blood circulation in the healthy muscles.

5
SAFETY WARNING
CONTRAINDICATIONS
Do not use this device on people who have a cardiac pacemaker, implanted defibrillator, or other implanted
metallic or electronic device, because this may cause electric shock, burns, electrical interference, or death.
Do not use this device on people whose pain syndromes are undiagnosed.
WARNINGS
• Use carefully. May cause serious burns. Do not use over sensitive skin areas or in the presence of poor
circulation. The unattended use of this device by children or incapacitated persons may be dangerous.
To reduce the risk of buns, electric shock, and fire, this device must be used in accordance with the
instructions.
• Do not crush the device and the wrap and avoid sharp folds.
• Carefully examine the device and the wrap, and do not use if they show any sign of deterioration.
• Do not tamper with this device and the wrap in any way. There are no user serviceable parts. If for any
reason they do not function satisfactorily, return to the authorized service center at address given.
• The long-term effects of chronic electrical stimulation are unknown.
• Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known
sensitivity to the carotid sinus reflex.
• Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal and pharyngeal
muscles may occur and the contractions may be strong enough to close the airway or cause difficulty in
breathing.
• Stimulation should not be applied transthoracically in that the introduction of electrical current into the
heart may cause cardiac arrhythmias.
• Stimulation should not be applied transcerebrally.
• Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g.,
phlebitis, thrombophlebitis, varicose veins, etc.
• Stimulation should not be applied over, or in proximity to, cancerous lesions.

6
PRECAUTIONS
• Safety of stimulation use during pregnancy has not been established.
• Caution should be used for people with suspected or diagnosed heart problems.
• Caution should be used for people with suspected or diagnosed epilepsy.
• Caution should be used if you have any of the following:
• if you have a tendency to bleed internally following an injury;
• if you recently had surgery, or have ever had surgery on your back;
• if areas of skin lack normal sensations, such as skin that is numb.
• Consult with your physician before use over the menstrual uterus.
• Caution should be used in the presence of the following:
• When there is a tendency to hemorrhage following acute trauma or fracture;
• Following recent surgical procedures when muscle contraction may disrupt the healing
process;
• Over the menstruating or pregnant uterus;
• Over areas of the skin which lack normal sensation.
• You may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical
conductive medium. The irritation can usually be reduced by using an alternate conductive medium,
or alternate electrode placement.
• Do not use this device while driving, operating machinery, or during any activity in which involuntary
muscle contractions may put the user at undue risk of injury.
• Keep this device out of reach of children.
• Do not use this device while sleeping.
• Do not use this device in high humidity areas such as a bathroom.
• Stop using this device at once if you feel discomfort, dizziness or nausea, and consult your physician.
• Do not attempt to move the conductive silver pads while the device is operating.
• Do not apply stimulation of this device in the following conditions:
• across the chest because the introduction of electrical current into the chest may cause
rhythm disturbances to the heart, which could be lethal;
• over open wounds or rashes, or over swollen, red, infected, or inflamed areas or skin
eruptions (e.g., phlebitis, thrombophlebitis, varicose veins);
• in the presence of electronic monitoring equipment (e.g., cardiac monitors, ECG alarms); on
children or incapacitated persons.

7
• Be aware of the following.
• consult with your physician before using this device;
• this device is not effective for pain associated with Central Pain Syndromes, such as
headaches;
• the device is not a substitute for pain medications and other pain management therapies;
• the device is a symptomatic treatment and, as such, suppresses the sensation of pain that
would otherwise serve as a protective mechanism;
• stop using the device if the device does not provide pain relief; and,
• use this device only with the conductive silver pads, and accessories recommended for
use by the manufacturer
• Keep the device away from high-temperature and direct-sunlight place.
• Do not share the use of the conductive silver pads with others.
• Do not use the device while it’s charging.
• The device contains the lithium battery. If overheating of the device occurred during the charging,
stop the charging or operation immediately and report to the seller.
• Dispose of the battery-containing device according to the local, state, or federal laws.
• Skin burns may occur, and check the skin under the conductive silver pads periodically.

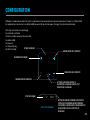
8
Consult instructions
for use
Catalogue number Humidity limitation
Caution, consult
accompanying documents
Serial number Non-sterile
Use by date Type BF applied Part Fragile, handle with care
Date of manufacture Manufacturer Keep away from rain
Batch code Temperature limitation
CE mark shows the
conformity to the Medical
Device Directive
Unrecyclable
IP22
IP code of the device
ADVERSE REACTIONS
• You may experience skin irritation and burns beneath the conductive silver pads applied to the skin;
• You should stop using the device and should consult with your physicians if you experience adverse
reactions from the device.
SYMBOL AND TITLE
• Information essential for proper use shall be indicated by using the corresponding symbols. The following
symbols may be seen on the device and labeling.

9
ENVIRONMENTAL CONDITION FOR TRANSPORT AND STORAGE
TEMPERATURE LIMITATION
5 °C
40 °C
Normal working ambient temperature: 5~40°C (41~104°F)
15 %
90 %
Normal working ambient humidity: 15~90%
-25 °C
70 °C
TEMPERATURE LIMITATION
Store and transport ambient temperature: -25~70°C (-13~158°F)
0 %
90 %
Store and transport ambient humidity: 0~90%
Fragile; handle with care
Keep away from rain
Non-sterile
Product packaging is able to be recycled
Atmospheric pressure: 70~106kPa

10
GUIDANCE AND MANUFACTURE’S DECLARATION – ELECTROMAGNETIC EMISSION
The device is intended for use in the electromagnetic environment specied below. The customer of the user of the device should
assure that it is used in such an environment.
Emission test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11
Group 1
The device uses RF energy only for its
internal function. Therefore, its RF emissions
are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emission
CISPR 11
Class B
Harmonic emissions
IEC 61000-3-2
Class A
The device is suitable for use in all
establishments, including domestic establish
public low-voltage power supply network
that supplies buildings used for domestic
purposes.
Voltage uctuations/ icker emissions
IEC 61000-3-3
Complies
ELECTROMAGNETIC COMPATIBILITY
• This product needs special precautions regarding electromagnetic compatibility (EMC) and needs to
be installed and put into service according to the EMC information provided. This unit can be affected
by portable and mobile radio frequency (RF) communications equipment.
• Do not use a mobile phone or other devices that emit electromagnetic fields, near the unit. This may
result in incorrect operation of the unit.
• Caution: This unit has been thoroughly tested and inspected to assure proper performance and
operation.
• Caution: This unit should not be used adjacent to or stacked with other equipment and that if
adjacent or stacked use is necessary, this unit should be observed to verify normal operation in the
configuration in which it will be used.

11
GUIDANCE AND MANUFACTURE’S DECLARATION – ELECTROMAGNETIC EMISSION
The device is intended for use in the electromagnetic environment specied below. The customer of the user of the device should
assure that it is used in such an environment.
Immunity test
IEC 60601-1-2
test level
Compliance level Electromagnetic environment - guidance
Electrostatic
discharge
(ESD)
IEC 61000-4-2
±8 kV contact
±2 kV, ±4kV, ±8 kV,
±15 kV air
±8 kV contact
±2 kV, ±4kV, ±8 kV,
±15 kV air
Floors should be wood, concrete or ceramic tile. If oor are
covered with synthetic material, the relative humidity should
be at least 30%.
Electrical fast
transient/
burst
IEC 61000-4-4
±2 kV for power
supply lines
±1 kV for input/
output lines
±2 kV for power supply
lines
±1 kV for input/output
lines
Main power quality should be that of a typical commercial or
hospital environment.
Surge
IEC 61000-4-5
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
Main power quality should be that of a typical commercial or
hospital environment.
Voltage
dips, short
interruptions
and voltage
variations on
power supply
input lines
IEC 61000-
4-11
0 % UT; 0.5 cycle
at 0°,45°,90°, 135°,
180°, 225°, 270°,
315°
0 % UT ; 1 cycle
70 % UT; 25/30 cycle
0% UT; 250/300
cycle
0 % UT; 0.5 cycle
at 0°,45°,90°, 135°,
180°, 225°, 270°,
315°
0 % UT ; 1 cycle
70 % UT; 25/30 cycle
0% UT; 250/300 cycle
Main power quality should be that of a typical commercial
or hospital environment. If the user of the device
requires continued operation during power nterruptions,
it is recommended that the device be powered from an
uninterruptible power supply or a battery.
Power
frequency
(50Hz/60Hz)
magnetic eld
IEC 61000-4-8
30 A/m 50Hz/60Hz 30 A/m 50Hz/60Hz
Main power quality should be that of a typical commercial
or hospital environment. If the user of the device requires
continued operation during power interruptions, it is
recommended that the device be powered from an
uninterruptible power supply or a battery.
NOTE UT is the a.c. mains voltage prior to application of the test level.

12
GUIDANCE AND MANUFACTURE’S DECLARATION – ELECTROMAGNETIC IMMUNITY
The device is intended for use in the electromagnetic environment specied below. The customer or the user of the device should
assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V RMS outside the
ISM band, 6 V RMS in
the ISM and amateur
bands
10 V/m
80 MHz ¨C 2,7 GHz 80
% AM at 1 kHz
3 Vrms
150 kHz to 80 MHz
3 V RMS outside
the ISM band, 6 V
RMS in the ISM and
amateur bands
10 V/m
80 MHz ¨C 2,7 GHz
80 % AM at 1 kHz
Portable and mobile RF communications equipment should
not be used near any part of the device, including cables, than
the recommended separation distance calculated from the
equation applicable to the frequency of the transmitter.
Recommended separation distance
d = 1,2 P
d = 1,2 P 80 MHz to 800 MHz
d = 2,3 P 80 MHz to 2,5 GHz
Where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation distance
in metres (m).
Field strengths from xed RF transmitters, as determined by
an electromagnetic site survey, a) should be less than the
compliance level in each frequency range. b)
Interference may occur in the vicinity of equipment marked
with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is aected by absorption and reection from
structures, objects and people.
a Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios,
amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the
electromagnetic environment due to xed RF transmitters, an electromagnetic site survey should be considered. If the measured eld
strength in the location in which the device is used exceeds the applicable RF compliance level.

13
observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting
or relocating the device.
b Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3 V/m.
THE DEVICE IS INTENDED FOR USE IN THE ELECTROMAGNETIC ENVIRONMENT SPECIFIED BELOW. THE CUSTOMER
OR THE USER OF THE DEVICE, SHOULD ASSURE THAT IT IS USED IN SUCH AN ENVIRONMENT.
Test Frequency
(MHz)
Band
a
Service
a
Modulation
a
Maximum
Power (W)
Distance (M)
Immunity Test
Level (V/m)
385 380-390 TETRA 400. Pulse Modulation
b
18Hz 1.8 0.3 27
450 430-470 GMRS 460. FRS 460. FM
c
+5kHz sine 2 0.3 28
710
704-787 LTE Band 13. 17.
Pulse Modulation
b
217 Hz
0.2 0.3 9745
780
810
800-960
GSM 800/900.
TETRA 800. iDEN 820.
CDMA 850. LTE.
Nand 5.
Pulse Modulation
b
18 Hz
2 0.3 28
870
930
1720
1700-1990
GSM 1800. CDMA.
1900.
GSM 1900. DECT.
LTE Nand 1, 3.
4, 25; UTMS.
Pulse Modulation
b
217 Hz
2 0.3 28
1845
1970
2450 2400-2570
Bluetooth WLAN.
802.11 b/g/n.
RFID 2450.
LTE Band 7.
Pulse Modulation
b
217 Hz
2 0.3 28
5240
5100-5800 WLAN 802. 11 a/n.
Pulse Modulation
b
217 Hz
0.2 0.3 95500
5785
NOTE If necessary to achieve the IMMUNITY TEST LEVEL, the distance between the transmitting antenna and the ME EQUIPMENT or ME SYSTEM may
be reduced to 1m. The 1m test distance is permitted by IEC 61000-4-3.
a) For some services, only the uplink frequencies are included
b) the carrier shall be modulated using a 50% duty cycle square wave signal.
c) As an alternative to FM modulation at 18 Hz may be used because while it does not represent actual modulation, it would be worst case.

14
HOW THE DEVICE WORKS
The device has multiple modes, covering TENS and EMS. If you are using the device for the first time, it is
recommended that you start with the default Mode 1, which combines different pulse frequencies. Some
modes are particularly effective for certain users, but you may need to select the mode that is best for you.
You may need to try a few modes before finding the one that suits you the best. The best choice of modes
may vary from one user to another, in spite of having the same type of symptom.
Pulse frequency (Hz) MODE DESCRIPTION INTENDED USE
Mode 1 Combination of Mode 2-8 Combination of below
Pain relief and muscle
stimulation with combined
pulse rates
Mode 2 69 Hz
Pulse on for 3.4 second
and off for 1.6 second
Pain relief with a pulse rate
of 69 Hz
Mode 3 12.5-55.5 Hz
Pulse on for 20 second
and off for 1 second
Muscle stimulation with
variable pulse rate
Mode 4 1.2 Hz
Pulse on every 0.85
second
Pain relief with a pulse rate
of 1.2 Hz
Mode 5 100 Hz
Pulse on for 10 second
and off for 2.5 second
Pain relief with a pulse rate
of 100 Hz
Mode 6 100 Hz
Pulse on for 20 second
and off for 1 second
Pain relief with a pulse rate of
100 Hz and longer on time
Mode 7 20 Hz
Pulse on for 5 second
and off for 1 second
Muscle stimulation with a fixed
pulse rate of 20 Hz
Mode 8 160 Hz
Pulse on for 10 second
and off for 2 second
Pain relief with a pulse rate
of 160 Hz

15
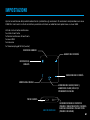
DEVICE DESCRIPTION
Remove the device and accessories from the packaging. The accessories include a USB cable used for
charging, and a wrap with conductive silver pads.
Accessories included in the package.
1x Tens unit controller
1x Wrap
1x USB line
1x Manual
1x Bottle
MICRO USB TO USB
CHARGER
INTENSITY INCREASE
HEAT LEVEL
INTENSITY DECREASE
MODE CHANGE
TIMER CHANGE
CHARGING PORT
BATTERY INDICATOR
ON / OFF: HOLD 2 SEC.
USE ON/OFF BUTTON TO
LOCK SCREEN

16
OPERATING INSTRUCTION
The following steps are used to guide the device operation, and the details about each step are listed in the
following table.
01 Charge control unit before first use
02 Lightly dampen conductive silver pads with water
03 Apply brace to body part
NOTE: Skin should be clean. Remove sweat and lotions prior to use.
04 Connect control unit to brace
Note: Connect gold magnets to gold connectors, silver magnets to silver connectors.
The screen should be legible from the user looking down.
05 Turn control unit on (Hold 2 sec.)
06 Select program
07 Change simulation time
08 Adjust stimulation intensity
09 Adjust heat level
10 Turn control unit off when done
NOTE: Intensity + button only works when device is attached to wrap and wrap is in
contact with skin.

17
1st Step – Charge control unit before rst use
CONTROL UNIT USB CABLE
The control unit comes with a built-in rechargeable battery, and can be
used as received. If the battery icon on the turned-on control unit keeps
ashing, it means the battery is running out. Charge the control unit with
the enclosed USB cable. The battery icon ashes during charging, and
becomes solid when the control unit is charged fully.
2nd Step - Dampen wrap pads for best results
Lightly dampen conductive silver pads with clean water
3rd Step - Apply to the body part
Apply wrap to the body part using velcro strap/s to secure in place
4th Step - Connect control unit to the brace
MAGNETS
Apply control unit to brace using magnets to secure connectors. Connect
gold magnets to gold connectors, and silver magnets to silver connectors.
The screen should be right reading from the user looking down.
5th Step - Turn control unit on
ON/OFF BUTTON
Hold On/O button for 2 seconds to turn on device. Front display panel will
light-up, indicating the device is on. Press the On/O button once to lock/
unlock the screen buttons. When buttons are locked, no adjustments can
be made. Hold On/O button for 2 seconds to turn o the device.

18
6th Step - Select program
M = MODE CHANGE
There are eight stimulation modes. Press the “M” button
to select a desired pulse mode. The mode selected will be
shown on the display.
7th Step - Change simulation time
T = TIMER CHANGE
Default timer is set to 30 min. Press the “T” button to select
a desired time (30, 40, 50, 60, 10 and 20 min) interval. The
time selected will be shown on the display screen.
8th Step - Adjust stimulation intensity
+ = INTENSITY INCREASE
- = INTENSITY DECREASE
Press and release the “+” button to increase the intensity,
and the “-” button to decrease the intensity. The device will
beep and the intensity level will ash with each change.
Note: With the increase of intensity (20 total levels), you
may experience sensations like tingling, vibration, pain,
etc. Therefore, gradually increase the intensity, and stop
increasing when a comfortable level is reached.
9th Step – Adjust heat level
HEAT + / - =
HEAT OFF, LEVEL 1, LEVEL 2
Press the HEAT +/- button to select desired level of heat.
10th Step - Turn control unit o when done.
OFF / ON
When the countdown timer is up, the device will turn o
automatically. The device can also be turned o by holding
the On/O button, indicated by the display light turning o.

19
When using the device for the first time, we recommend starting from the default Mode 1, which combines
the different frequencies. If you use one of the specific TENS or EMS modes, please refer to the following
for details.
USE AS TENS
The control unit includes the following TENS frequencies, 69Hz (Mode 2), 1.2 Hz (Mode
4), 100Hz (Modes 5 and 6), and 160Hz (Mode 8). For treatment, place the wrap and conductive silver pads
at the site of pain, and connect the device to the wrap.
For arthritis pain, inflammation of the joints, place the conductive silver pads on or near the area of
the arthritis pain. The TENS mode of the device generates electrical pulses that are sent through the
conductive silver pads for pain relief.
USE AS EMS
The control unit also includes the EMS frequencies, 12.5-55.6Hz (Mode 3) and 20Hz (Mode 7). For
treatment, place the wrap and conductive silver pads where you desired muscle firming and strengthening,
and connect the device to the wrap. Then select modes 3 or 7.
Recommended Practice for Both TENS and EMS:
Start from the lowest intensity and gradually adjust the intensity to a comfortable level at a scale from
from 1 to 20.
Good skin care is important for a comfortable use of device. Be sure the treatment site is clean of dirt and
body lotion.

20
CONDUCTIVE WRAP
The conductive wrap accessory is connected to the control unit through its magnentic connectors. The
wrap accessory holds the conductive silver pad material that will be in contact with the skin. The electrical
stimulation will be delivered to the body through these pads.
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
-
 65
65
-
 66
66
-
 67
67
-
 68
68
-
 69
69
-
 70
70
-
 71
71
-
 72
72
-
 73
73
-
 74
74
-
 75
75
-
 76
76
-
 77
77
-
 78
78
-
 79
79
-
 80
80
-
 81
81
-
 82
82
-
 83
83
-
 84
84
-
 85
85
-
 86
86
-
 87
87
-
 88
88
-
 89
89
-
 90
90
-
 91
91
-
 92
92
-
 93
93
-
 94
94
-
 95
95
-
 96
96
-
 97
97
-
 98
98
-
 99
99
-
 100
100
-
 101
101
-
 102
102
-
 103
103
-
 104
104
-
 105
105
-
 106
106
-
 107
107
-
 108
108
-
 109
109
-
 110
110
-
 111
111
-
 112
112
-
 113
113
-
 114
114
-
 115
115
-
 116
116
-
 117
117
-
 118
118
-
 119
119
-
 120
120
-
 121
121
-
 122
122
-
 123
123
-
 124
124
-
 125
125
-
 126
126
-
 127
127
-
 128
128
-
 129
129
-
 130
130
-
 131
131
-
 132
132
-
 133
133
-
 134
134
-
 135
135
-
 136
136
-
 137
137
-
 138
138
-
 139
139
-
 140
140
-
 141
141
-
 142
142
-
 143
143
-
 144
144
-
 145
145
-
 146
146
-
 147
147
-
 148
148
-
 149
149
-
 150
150
-
 151
151
-
 152
152
-
 153
153
-
 154
154
-
 155
155
-
 156
156
-
 157
157
-
 158
158
-
 159
159
-
 160
160
-
 161
161
-
 162
162
-
 163
163
-
 164
164
-
 165
165
-
 166
166
-
 167
167
-
 168
168
-
 169
169
-
 170
170
-
 171
171
-
 172
172
-
 173
173
-
 174
174
-
 175
175
-
 176
176
-
 177
177
-
 178
178
-
 179
179
-
 180
180
-
 181
181
-
 182
182
-
 183
183
-
 184
184
-
 185
185
-
 186
186
-
 187
187
-
 188
188
-
 189
189
-
 190
190
-
 191
191
-
 192
192
-
 193
193
-
 194
194
-
 195
195
-
 196
196
-
 197
197
-
 198
198
-
 199
199
-
 200
200
-
 201
201
-
 202
202
-
 203
203
-
 204
204
-
 205
205
-
 206
206
-
 207
207
-
 208
208
-
 209
209
-
 210
210
-
 211
211
-
 212
212
-
 213
213
-
 214
214
-
 215
215
-
 216
216
-
 217
217
-
 218
218
-
 219
219
-
 220
220
-
 221
221
-
 222
222
-
 223
223
-
 224
224
-
 225
225
-
 226
226
-
 227
227
-
 228
228
-
 229
229
-
 230
230
-
 231
231
-
 232
232
-
 233
233
-
 234
234
-
 235
235
-
 236
236
-
 237
237
-
 238
238
-
 239
239
-
 240
240
-
 241
241
-
 242
242
-
 243
243
-
 244
244
Compex Back Wrap for Pain Relief Manuel utilisateur
- Taper
- Manuel utilisateur
- Ce manuel convient également à
dans d''autres langues
- italiano: Compex Back Wrap for Pain Relief Manuale utente
- English: Compex Back Wrap for Pain Relief User manual
- español: Compex Back Wrap for Pain Relief Manual de usuario
- Deutsch: Compex Back Wrap for Pain Relief Benutzerhandbuch
- Nederlands: Compex Back Wrap for Pain Relief Handleiding
- português: Compex Back Wrap for Pain Relief Manual do usuário
- dansk: Compex Back Wrap for Pain Relief Brugermanual
- svenska: Compex Back Wrap for Pain Relief Användarmanual
- suomi: Compex Back Wrap for Pain Relief Ohjekirja
Documents connexes
Autres documents
-
Thule Artificial Raingutters Manuel utilisateur
-
Drive Medical Padded Swivel Seat Cushion Manuel utilisateur
-
Terraillon Trio Care Le manuel du propriétaire
-
Terraillon Trio Care Mode d'emploi
-
Beurer EM 44 Le manuel du propriétaire
-
Omron PM3032 Manuel utilisateur
-
Terraillon Easy Care Le manuel du propriétaire
-
Beurer S02961 Mode d'emploi
-
 Pressalit M60234 Mode d'emploi
Pressalit M60234 Mode d'emploi
-
Moretti RM370 Manuel utilisateur
























































































































































































































































