Therabody 15225120 Mode d'emploi
- Catégorie
- Moniteurs de fréquence cardiaque
- Taper
- Mode d'emploi

TheraFace PRO
EN/DE/FR/ES

TABLE OF CONTENTS
Language
EN 2- 21
DE 22-41
FR 42-61
ES 62-81

2
EN
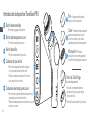
Product Overview
TheraFace PRO
An advanced solution for better facial wellness.
The TheraFace PRO is a 4-in-1 handheld device that takes facial wellness to the next level by helping to reduce tension,
relax facial muscles, and achieve healthier-looking skin by gently stimulating the face. It includes microcurrent, blue, red,
and red+infrared light LED, and cleansing treatments, allowing you to customize your facial therapy in one easy-to-use
versatile device. Use the variety of treatment rings to help lift, tone, rejuvenate, and deep clean. The TheraFace PRO is
your all-in-one solution for optimal health and wellness for the face.
Intended Use
Percussive Therapy
The TheraFace PRO is intended to help reduce tension, relax facial muscles, and achieve more beautiful skin by gently
stimulating the face. The variety of treatment rings are intended to help lift, tone, rejuvenate, and deep clean.
Percussive therapy, now optimized for the face. Facial massage to reduce tension and relax facial muscles.

3
EN
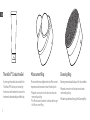
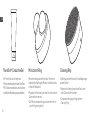
Microcurrent Ring Cleansing Ring
Microcurrent firms and tightens the skin. Microcurrent
improves muscle tone and contour in the face/neck
0 Magnetic connection to the device and can be
removed by pulling
0 The Microcurrent treatment is delivered through
the Microcurrent Ring
A primer gel intended to be used with the
TheraFace PRO device prior to starting
the microcurrent treatment to ensure the
treatment is delivered safely and effectively
Cleansing removes facial build up of dirt, oil, and debris
0 Magnetic connection to the device and can be
removed by pulling
0 All cleansing is delivered through the Cleansing Ring
TheraOneTM Conductive Gel

4
EN
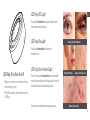
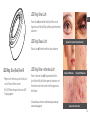
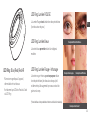
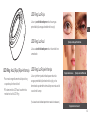
LED Ring: Blue/Red/Red+IR LED Ring: Red + Infrared Light
Red + Infrared Light is intended to reduce periorbital
wrinkles (the wrinkles around the eyes) and is shown to
increase the body’s natural healing process
LED Ring: RED Light
Red Light is intended to reduce periorbital wrinkles
(the wrinkles around the eyes)
LED Ring: Blue Light
Blue Light is intended to reduce mild to
moderate acne
*Blue Light is not intended to treat or reduce severe acne
Example of Periorbital Wrinkles
Example of Mild Acne Example of Moderate Acne
Example of Severe Acne*
0 Magnetic connection to the device and can
be removed by pulling
0 The LED treatment is delivered through the
LED Ring

5
EN Getting To Know Your TheraFace PRO Device
Percussive Therapy Button
0 Controls the Percussive Attachment
Ring Button
0 Controls the Attachment Ring
Attachment Ring
0 Magnetic connection to the device and
can be removed by pulling
0 All the Attachment Rings have only one
correct position
Percussive Therapy Attachments
0 Magnetic connection to the device and
can be removed by pulling
0 All the Attachment Rings have only one
correct position
Power Button
0 Powers the device ON / OFF
Flat - General use for the entire
face, neck and chest
Cone - More precise treatment
for targeted areas such as around
the eyes, nasolabial lines and
pressure points
Micropoint - Maximize
circulation for larger areas such as
the forehead, cheeks and chest
Hot and Cold Rings
(sold separately)
0 Magnetic connection to the device
and can be removed by pulling
0 Delivers the option of 3 Cold and
3 Hot settings

6
EN
Getting Started
Push and hold the ON/OFF button to power on the
device and the OLED screen will turn on.
Powering on the TheraFace PRO device
1. Start with a clean, dry face
2. Select and attach your desired
attachment ring
3. Turn on the device
4. Choose your settings
5. Get started
Basic getting started steps:
Push and hold the ON/OFF button again to switch the
device off and the OLED screen will be off.
Switching O
If the device is not manually powered off by pressing the Power button, the
device will automatically shut off after 10 minutes of inactivity.
Automatic Shut O Note: Make sure you turn off
the power on the device before
removing the rings.

7
EN B. LED Treatment
1. The LED ring has 3 light therapy wavelength options: red, blue and
red+infrared.
2. To start using the LED therapy mode, attach the LED ring to the
TheraFace PRO device.
3. Turn ON the TheraFace PRO device by pressing the Power Button
for 2 seconds.
4. Then, turn ON the LED ring by pressing the Attachment Ring button.
5. To toggle between light options (blue, red and red+infrared), press
the Attachment Ring button again.
6. The TheraFace PRO LED ring has a proximity sensor system, and will
only reach full light intensity (brightness) once the device is placed at
the right distance from your face (0.5 inches), reducing glare in the
interim. Avoid direct skin contact.
7. To turn off the LED ring, toggle to the Red+IR light mode and
press the Attachment Ring Button one more time or power off the
TheraFace PRO device by pressing the Power Button for 2 seconds.
Warning: Do not place the LED Ring directly on the skin. Keep the LED
Ring approximately 0.5 inches away from the skin.
Using the TheraFace PRO
Start by washing your face completely and rinse twice to remove residue.
Allow skin to dry completely before starting treatment. Be sure to visit
therabody.com for treatment protocols and instructional videos.
A. Percussive Therapy Feature
1. Select and attach the desired percussive therapy attachment
2. Once attached to the TheraFace PRO, turn ON the TheraFace PRO
device by pressing the Power Button for 2 seconds.
3. Turn on the percussive feature by pressing the percussive button once.
4. To toggle between the 3 speed options (1750, 2100 and 2400 rpm), press
the percussive button again.
5. To stop the percussive treatment, press the percussive button a third time.
Note: • Can be combined with Hot and Cold Rings* - (*Hot and Cold Rings sold separately)
• Cannot be combined with the Microcurrent attachment or with the LED ring
Note: The TheraFace PRO device
will make a beeping sound every 15
seconds. This will help you track the
time within each treatment and will
also inform you if a treatment has
been disconnected or is completed.
• 1 beep = timed feedback every 15 sec so you
can time their protocols better
• 2 beeps = the LED, Microcurrent, Hot, or Cold
treatment has been accidentally disconnected
• 3 beeps = the LED, Microcurrent, Hot, or Cold
treatment is over

8
EN
B. LED Treatment C. Microcurrent Treatment
1. Remove any percussive therapy attachment before attaching the
Microcurrent Ring.
2. Prior to starting the microcurrent treatment, apply the TheraOne™
Conductive Gel to your clean, dry face or treatment area to ensure
the treatment is delivered correctly.
3. Attach the Microcurrent Ring to the TheraFace PRO device.
4. Once attached to the TheraFace PRO, turn ON the TheraFace PRO
device by pressing the Power Button for 2 seconds.
5. Turn on the microcurrent treatment by pressing the ring button once.
6. To adjust the intensity level to your comfort, press the ring button once
to toggle between the 3 intensity options.
7. To stop the microcurrent treatment, press the ring button a third time.
Warning: When using the TheraFace PRO with the Microcurrent Ring, a
microcurrent primer is required. Do NOT use the Microcurrent Ring on the midline
of the neck or eye area.
PRO Tip: When using the Microcurrent Ring, slowly glide the device using light
to medium pressure, keeping both spheres on the face at once. A microcurrent
protocol of 5-8 minutes can be completed once per 24-hour period. Be sure to
visit therabody.com for treatment protocols and instructional videos.
The TheraOne™ Conductive Gel is a primer intended to be used with the
TheraFace PRO device prior to starting the microcurrent treatment to ensure
the treatment is delivered safely and eectively.
D. Microcurrent Primer: TheraOne Conductive Gel
Directions:
1. Start with a clean, dry face.
2. Apply a mask-like layer of the conductive gel to all treatment areas
of the face and neck.
3. Following treatment with the microcurrent ring, rinse off and apply
your favorite moisturizer.
Note: • There is a preset shut off time of 8 minutes
• Cannot be combined with the percussive therapy attachments
• While the Microcurrent Ring will work with any conductive gel, we
recommend the TheraOne™ Conductive Gel if available in your market.

9
EN
1. Tone: Use one cotton pad with toner applied sweeping gently
across the face, avoiding the eye area and concentrating on the
nose, nasal fold, and chin
2. Serum: Apply serum
3. Moisturizer: Apply moisturizer
4. SPF: Apply SPF only in the morning or daytime hours
If you already use a toner, serum, moisturizer, and/or SPF, then apply in
this order following the completion of any TheraFace PRO protocol:
After Skin Treatment
Clean your face with a warm, damp washcloth.
Device Maintenance
The following maintenance instructions are important to ensure that
your device continues to work as it was designed. Failure to follow these
instructions may cause your device to stop working.
Care and Cleaning
Visually inspect the TheraFace PRO device and attachment rings for any obvious
signs of debris build-up. Wipe your device and attachment rings down with a damp
cloth or alcohol-free cleansing wipe. After cleaning, allow the device and attachment
rings to dry thoroughly before storing or beginning another treatment protocol. A
properly cleaned device should have no visible signs of debris or moisture.
Note: This product is not waterproof, only clean with a damp cloth or alcohol-free cleansing
wipe by wiping the device and attachment rings. Do not submerge the device in water or clean
it under running water (other than the Cleaning Ring). Do not allow the device to come into
contact with any corrosive solutions, which would damage appearance and function. Store
the device in a cool and dry place (Temperature: 0°C/32°F - 40°C/104°F Relative humidity:
10~95% RH). Do not store the device or battery where temperatures may exceed 40°C/104°F,
such as in direct sunlight or in a vehicle.
TheraFace PRO Device After Care & Cleaning
Warnings: Use only as directed. Avoid eye contact. Do NOT use the on the midline
of the neck. Prior to starting the microcurrent treatment, test by applying a small
amount of the TheraOne Conductive Gel on a small patch of skin rst. Skin may
absorb gel during microcurrent session; reapply if needed to ensure even glide of
device on the skin.

10
EN
Charging the TheraFace PRO
• The TheraFace PRO is USB-C enabled with the connector at the bottom of the
device.
• The TheraFace PRO includes a USB-C to USB-A cable.
• You can plug the device into any standard USB adapter.
• The TheraFace PRO allows for fast-charging if charged with a USB-C adapter.
Note: Make sure the charger is from a certified manufacturer and it hasn’t suffered any
structural damage. The TheraFace PRO device will not work while charging.
TheraFace PRO Warnings and Guidance
(Precautions and Contraindications)
Background
This device is intended to be used on the face, neck, and upper chest regions of
the body. If you have any specific medical conditions or concerns, please consult
your physician before using this product. There will be times when it is advisable
to modify how devices are used (precautions) or times when it is not appropriate to
use certain devices (contraindications). The following document highlights these
for each modality or treatment attachment included with the TheraFace PRO
device as of the date of printing. For up-to-date information, please visit us online.
Important Safety Information
General TheraFace PRO Use
Read the full Warnings and Guidance prior to using the TheraFace PRO.
This device is contraindicated against and should not be used by or on anyone with a history
of epilepsy, seizures or cardiopathy. The TheraFace PRO is not recommended for anyone
with an electronic implanted device (such as a pacemaker), cardiac arrhythmia, tumors, or
acute episodes of inflammatory diseases. The device is not recommended for those who have
arteriosclerosis, thromboses or implants in the body region being treated. Dental implants
should be firmly anchored before using the device. Do not use the device on the eyes, eyelids,
or the area immediately surrounding the eye (Periorbita area). The device should not be used
if you have dark brown or black spots, such as large freckles, birthmarks, moles or warts, on the
area to be treated. The device is not recommended if you have eczema, psoriasis, lesions, open
wounds or active infections other than mild to moderate acne, such as cold sores, in the area to
be treated. Wait for the infected area to heal before using the device. The device should not be
used if you have abnormal skin conditions caused by diabetes or other systemic or metabolic
diseases. It is not recommended to use this device if you have a history of herpes outbreaks
in the area of treatment, unless you have consulted with your physician and have received
preventive treatment before using the device. Please consult your physician prior to use if you
are pregnant and/or nursing.
Please consult your physician prior to use if you are pregnant and/or nursing.
Immediately stop using the device at the first sign of discomfort.
The device is not recommended for anyone under the age of 18 without adult supervision
and should be kept out of reach of children. If you have any medical concerns, are taking
any medications that cause light sensitivity, or have had any facial surgery or other surgical
procedures, please consult your doctor before using the device.

11
EN Microcurrent Ring Attachment
These recommendations are derived from consultation with
medical experts and the published research in regards to
precautions and contraindications and are as of the date of
printing. For up-to-date information, please visit us online.
When using the device with the microcurrent treatment,
a microcurrent conductive gel primer is required. Prior to
starting the microcurrent treatment, test by applying a small
amount of the conductive gel on a small patch of skin.
Precautions
• Recent injury, surgery, or facial treatment such as neurotoxin,
dermal filler, microneedling, laser, and/or chemical peel until
skin has fully healed
• Do not use during a breakout from Herpes Simplex Virus
• Do not use over facial hair; facial hair must be shaved prior to
use as hair can interfere with the conductivity
• Do not use if you suffer from any heart condition
• Do not use directly over center of neck (bone), specifically
avoiding the thyroid
• Do not use on breast area
• Do not use across your chest
• Do not use on groin area
• Do not use directly on the eyes, eyelids, or the area immediately
surrounding the eye (Periorbita area).
• Do not apply to broken skin
• Do not use on children
Contraindications
The following are circumstances where the potential risks
may outweigh the benefits. Consult a medical professional
before use.
• Skin rash, open wounds, blisters, local tissue inflammation,
infections, bruises, or tumors
• Pacemaker or other implanted electronic devices
• Epilepsy
• Pregnancy
• Cancer/tumors
• Thrombosis
• Phlebitis
• Metal plates or pins in the application area
• Implanted defibrillators/ stimulators
LED Ring Attachment (Red
LED, Red+IR LED, and Blue LED
Therapies)
These recommendations are derived from consultation with
medical experts and the published research in regards to
precautions and contraindications as of the date of printing.
For up-to-date information, please visit us online.
Precautions
• Recent injury, surgery, or facial treatment such as neurotoxin,
dermal filler, microneedling, laser, and/or chemical peel until skin
has fully healed.
• Current breakout from Herpes Simplex Virus
• For facial hair: Use LED over facial hair, following the pattern of
hair growth (typically in a downward motion)
• Do not apply directly to the eyeball/eyelid
• Do not apply to broken skin
• Do not apply retinol before use of red LED light
Contraindications
The following are circumstances where the potential risks may
outweigh the benefits. Consult a medical professional before use.
• Skin rash, open wounds, blisters, local tissue inflammation,
infections, bruises, or tumors
• Pregnancy/nursing
• Abnormal sensations (ex. numbness)
• Cancer/tumors
• Epilepsy
• Cardiopathy (heart disease)
• Photo allergy or disorder (ex. Lupus, porphyria)
• Medications that cause light sensitivity
• Medications for severe acne
• Extreme sensitivity to light
• Melasma or hyperpigmentation (especially if exacerbated by
mild warmth)
• Suspicious lesions or skin cancer–please visit your physician
• If taking or using any retinol or sun-sensitive medications or
products or benzoyl peroxide do not use infrared light

12
EN
Percussive Therapy Attachments
(Flat, Cone, Micro-point)
These recommendations are derived from consultation with
medical experts and the published research in regards to
precautions and contraindications as of the date of printing.
For up-to-date information, please visit us online.
Precautions
Due care is required in these circumstances and the use of the
devices may need to be modified (such as attachment used,
the force applied, the position of the body, avoidance of use in
direct contact with an area, etc.). Where appropriate or if you
have any concerns, seek the advice of a medical professional
• Recent injury, surgery, or facial treatment such as neurotoxin,
dermal filler, microneedling, laser, and/or chemical peel until
skin has fully healed.
• Current breakout from Herpes Simplex Virus
• Hypertension (controlled)
• Abnormal sensations (e.g., numbness)
• Sensitivity to pressure
• Medications that may alter sensations
• Do not apply directly to the eyeball/eyelid
• Do not apply to broken skin
Contraindications
The following are circumstances where the potential risks may
outweigh the benefits. Consult a medical professional before use.
• Skin rash, open wounds, blisters, local tissue inflammation,
infections, bruises, or tumors
• Active acne breakout
• Bone fracture or myositis ossificans
• Hypertension (uncontrolled)
• Acute or severe cardiac, liver, or kidney disease
• Neurologic conditions resulting in loss or altered sensation
• Direct application to the eyes or throat
• Bleeding disorders
• Recent surgery or injury
• Connective tissue disorders
• Peripheral vascular insufficiency or disease
• Medications that thin the blood or alter sensations
• Direct pressure over surgical site or hardware
• Extreme discomfort or pain
• Pacemaker, ICD, or history of embolism
Cleansing Ring Attachment
These recommendations are derived from consultation with
medical experts and the published research in regards to
precautions and contraindications as of the date of printing.
For up-to-date information, please visit us online.
Precautions
• Recent injury, surgery, or facial treatment such as neurotoxin,
dermal filler, microneedling, laser, and/or chemical peel until skin
has fully healed.
• Current breakout from Herpes Simplex Virus
• If you have facial hair, use the cleansing ring following the pattern
of hair growth (typically a downward motion) and/or make small
circular motions if comfortable
• Do not apply directly to the eyeball/eyelid
• Do not apply to broken skin
Contraindications
The following are circumstances where the potential risks may
outweigh the benefits. Consult a medical professional before use.
• Skin rash, open wounds, blisters, local tissue inflammation,
infections, bruises, or tumors
Hot Ring Attachment
These recommendations are derived from consultation with
medical experts and the published research in regards to
precautions and contraindications as of the date of printing. For
up-to-date information, please visit us online.
Precautions
• Recent injury, surgery, or facial treatment such as neurotoxin,

13
EN dermal filler, microneedling, laser, and/or chemical peel until
skin has fully healed.
• Current breakout from Herpes Simplex Virus
• Do not apply directly on the eyeball/eyelid
• Do not apply to broken skin
Contraindications
The following are circumstances where the potential risks may
outweigh the benefits. Consult a medical professional before use.
• Skin rash, open wounds, bliszters, local tissue
inflammation, infections, bruises, or tumors
Cold Ring Attachment
These recommendations are derived from consultation with
medical experts and the published research in regards to
precautions and contraindications.
Precautions
• Recent injury, surgery, or facial treatment such as neurotoxin,
dermal filler, microneedling, laser, and/or chemical peel until
skin has fully healed.
• Current breakout from Herpes Simplex Virus
• For facial hair use Cold Ring as noted in standard protocol
• Do not apply on the eyeball/eyelid
• Do not apply to broken skin
Contraindications
The following are circumstances where the potential risks may
outweigh the benefits. Consult a medical professional before use.
• Skin rash, open wounds, blisters, local tissue inflammation,
infections, bruises, or tumors
• Cold hypersensitivity/Cold urticaria
• Circulatory insufficiency
Additional Unit Warnings
When using the device, these basic precautions should
always be followed:
1. USE ONLY AS INSTRUCTED. Use the device as described
in the device User Manual only. Only use the recommended
attachments, accessories, and replacement parts. Do not carry
out any maintenance on your own.
2. NOT FOR CHILDREN. The device is not intended for use by
young children or persons with reduced physical, sensory, or
reasoning capabilities, or lack of experience and knowledge
with how the device should be operated, unless they have
been given supervision or instruction by a responsible person.
Do not allow the device to be used as a toy. Children should be
supervised to ensure that they do not play with the device.
3. CHARGING LOCATIONS. The device should be charged
indoors in a well ventilated, dry location. Do not charge the
device outdoors, in a bathroom, or within 10 feet (3.1 meters)
of a bathtub or pool. Do not use the device or charger on wet
surfaces and do not expose the charger to moisture, rain, or snow.
Do not use the device in the presence of explosive atmospheres
(gaseous fumes, dust, or flammable materials). Sparks may be
generated, possibly causing a fire.
4. DO NOT OVERCHARGE. Do not leave the battery in the
Charger for more than 1 hour after the battery has been fully
charged. The battery includes a Theragun Protection system
to avoid the risk of overcharging. However, overcharging may
reduce its life over time
5. DO NOT BURN OR INCINERATE THE DEVICE OR ITS
BATTERIES. The battery may explode, causing personal injury
or damage. Toxic fumes and materials are created when the
battery is burned.
6. DO NOT CRUSH, DROP, OR DAMAGE THE DEVICE
BATTERIES OR CHARGER. Do not use a charger that has
received a sharp blow, been dropped, run over, or damaged in
any way.
7. BATTERY CHEMICALS CAUSE SERIOUS BURNS. Never allow
the internal battery to come into contact with the skin, eyes, or

14
EN
mouth. If a damaged battery leaks chemicals, use rubber or
neoprene gloves to dispose of it. If skin is exposed to battery
fluids, wash with soap and water and rinse with vinegar. If eyes
are exposed to battery chemicals, immediately flush with
water for 20 minutes and seek medical attention. Remove and
dispose of contaminated clothing.
8. DO NOT SHORT CIRCUIT. A battery will short circuit if a metal
object makes a connection between the positive and negative
contacts on the battery or the 16V connector. Do not place a
battery near anything that may cause a short circuit, such as
coins, keys, or nails in your pocket. A short circuited battery
may cause fire and personal injury.
9. DO NOT OPERATE UNDER BLANKET AND PILLOW OR
BETWEEN COUCH CUSHIONS. Excessive heating can occur
and cause fire, electric shock, or injury.
10. STORING THE DEVICE & BAT TERY. Store in a cool, dry place.
Only charge the device when the ambient temperature is
between 0°C/32°F - 40°C/104°F. Do not store the device or
batteries where temperatures may exceed 40°C/104°F, such as in
direct sunlight or in a vehicle.
11. DISPOSING OF BATTERIES. The TheraFace PRO lithium-ion
batteries for the device are more environmentally friendly
than some other types of batteries. Always dispose of device
batteries according to federal, state, and local regulations.
Contact a recycling agency in your area for recycling locations.
Even discharged batteries contain some energy. Before
disposing, use electrical tape to cover the device’s 16V
connector/terminals to prevent the battery from shorting,
which could cause a fire or explosion.
12. DO NOT DISASSEMBLE. Disassembly or incorrect reassembly
may result in the risk of electric shock, fire, or exposure to
battery chemicals. The warranty will be void if the device,
batteries, or charger are disassembled or if any parts have
been removed.
13. SERVICE. If the device, batteries, or charger is not working
properly, has received a sharp blow, or has been dropped,
damaged, left outdoors, or dropped into water, then do not use
it. Do not attempt to repair or disassemble the device which
may result in an electric shock or fire.
14. DO NOT USE WHILE BATHING OR IN THE SHOWER, TUB,
OR SINK. Do not place or store the device or batteries where
it can fall or be pulled into a tub or sink. Do not place in or
drop into water or other liquid. Do not reach for an appliance
that has fallen into or come into contact with water. Unplug
immediately.
15. THERMAL LIMITER. The device has an automatic reset thermal
limiter that shuts off the device to prevent overheating and fire.
16. POLARIZED PLUG. To reduce the risk of electric shock, this
appliance has a polarized plug (one blade is wider than the other).
This plug will fit in a polarized outlet only one way. If the plug
does not fit fully in the outlet, reverse the plug. If it still does not
fit, contact a qualified electrician to install the Theragun Proper
outlet. Do not change the plug in any way.
17. DO NOT OPERATE the device where aerosol (spray) is being
used or where oxygen is being administered.
SAVE THESE INSTRUCTIONS
Risk
1. Use the TheraFace PRO device and attachment rings only as
described within this User Manual. The risks and dangers of using
the TheraFace PRO device and attachment rings in any way other
than specified in the provided User Manual are unknown and may
result in negative side effects.
2. This TheraFace PRO device was not tested for use over the eye
socket or eyelid so the risks are unknown.

15
EN Labels
NO. SYMBOLS DESCRIPTION
1CE mark
2the Restriction of Hazardous Substances
3“WEEE (Waste Electrical and Electronic Equipment)”.
The waste products should be handled legally.
4Keep dry
5FCC Federal Communications Commission
6Please read the user manual before use
7Type BF apply part.
Product Specifications
BASIC UNIT CHARACTERISTICS
Power Source User supplied USB-A or USB-C adapter
Indicator Light YES
Housing Materials PC
ADDITIONAL FEATURES
Environment for operation Temperature: 0 ~ 40° C
Relative humidity: <93% RH
Environment for storage Temperature: -25° C ~ 50° C
Relative humidity: 10~95% RH
Use atmospheric pressure 70-106Kpa

16
EN
Indicator Light Yes , OLED display
Timer Range 5-8 minutes per day for 6 weeks
Housing Materials Console: PC plastic
OUTPUT SPECIFICATION
Waveform Pulsed Biphasic
Operating voltage 0-15.5V
Maximum power 24mW
Automatic shut-down Yes
Maximum Output
Voltage(+/- 10%)
210-280mV @500 Ohm
0.8- 1.2V @ 2k Ohm
4.75-5.2V @ 10k Ohm
For Microcurrent Treatment:
Stimulated site Face and neck
Number of modes 3
Output Intensity Level 4
Maximum Output Current 420 μA - 560 μA @ 500 Ohm
400 μA - 600 μA @ 2k Ohm
475 μA - 520 μA @ 10k Ohm
Pulse Width On 60ms / O 60ms
Frequency 8.3Hz
Net Charge N/A - Battery operated
Maximum Current Density 1.65mA/cm^2 @ 500 Ohm
Maximum Power Density 1.36125mW/cm² @ 500Ω
FOR LED TREATMENT
Light wavelength IR+Red:830nm±10nm/633 ± 10 nm
Blue light:415nm±10nm, Red light:633nm±10nm
Power of light
(mW//cm2) IR+Red 70±5%/60±5%
Red light 60±5%, Blue light 45±5%
FOR PERCUSSIVE THERAPY
Percussive 1750, 2100 and 2400 rpm
Frequency 1750rpm :29.1Hz 2100rpm:35Hz 2400rpm:40Hz

17
EN
1. This device is Class II equipment with type BF applied part. It complies with Medical
Electrical Safety Standards (IEC 60601-1).
2. This device is also compliant with Medical EMC Standard (IEC 60601-1-2).
3. All the user directly contracting materials for the main device housing and output
contacts in this device are biocompatible for its intended use. They comply with
biocompatibility standards ISO 10993-5 (Cytotoxicity) and ISO 10993-10 (Irritation
and Sensitization).
Safety, EMC & Bio-compatibility
CAUTION:
Do not apply the device near any devices with Electromagnetic Interference (EMI),
such as cell phones, Magnetic Resonance Imaging (MRI), computerized axial
tomography (CT), diathermy, Radio Frequency Identification (RFID), etc. or MR
environment. EMI, RF devices or MR environment may affect the normal function of
the device or would cause user injury.
The TheraFace PRO has been tested and found to comply with the electromagnetic
compatibility (EMC) limits for medical device to IEC 60601-1-2: 2007. These limits
are designed to provide reasonable protection against harmful interference in a
typical medical installation.
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) this device may not cause harmful interference, and (2) this device must accept
any interference received, including interference that may cause undesired operation. Changes
or modifications not expressly approved by the party responsible for compliance could void the
user’s authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class B digital device,
pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation. This equipment generates, uses and can
radiate radio frequency energy and, if not installed and used in accordance with the instructions,
may cause harmful interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation.
If this equipment does cause harmful interference to radio or television reception, which can be
determined by turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
FCC compliance statement
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the receiver is
connected.
• Consult the dealer or an experienced radio/TV technician for help.

18
EN
MANUFACTURER’S DECLARATION – ELECTROMAGNETIC EMISSIONS
The TheraFace PRO is intended for use in the electromagnetic environments specified below. The customer or the user of the TheraFace PRO should ensure that it is used in such an environment.
EMISSIONS TEST COMPLIANCE ELECTROMAGNETIC ENVIRONMENT - GUIDANCE
RF emissions
CISPR 11 Group 1 The TheraFace PRO uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment.
RF emissions
CISPR 11 Class B The TheraFace PRO is suitable for use in all establishments, including domestic establishments and those directly connected to
the public low-voltage power supply network that supplies buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2 Class A
Voltage function / flicker emissions
IEC 61000-3-2 Complies

19
EN
The TheraFace PRO is intended for use in the electromagnetic environment specified below. The customer or the user of the TheraFace PRO should ensure that it is used in such an environment.
IMMUNITY TEST IEC 60601 TEST LEVEL COMPLIANCE LEVEL ELECTROMAGNETIC ENVIRONMENT - GUIDANCE
Electrostatic discharge (ESD)
IEC 61000-4-2 ±6kV contact
±8kV air ±6kV contact
±8kV air Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the
relative humidity should be at least 30%.
Electrostatic transient / burst
IEC 61000-4-4 ±2kV for power supply lines
±1kV for input/output lines ±2kV for power supply lines
±1kV for input/output lines Mains power quality should be that of a typical commercial or hospital environment.
Surge
IEC 61000-4-5 ±1kV differential mode
±2kV common mode ±1kV differential mode
±2kV common mode Mains power quality should be that of a typical commercial or hospital environment.
Voltage dips, short interruptions and
voltage variations on power supply
input lines
IEC 61000-4-11
< <5% UT (><95% dip in UT)
for 5 sec <5% UT (><95% dip in UT) for 5 sec Mains power quality should be that of a typical commercial or hospital environment. If the
user of the TheraFace PRO requires continued operation during power mains interruptions, it is
recommended that the TheraFace PRO be powered from an uninterruptible power supply
or a battery.
Power frequency (50/60 Hz
magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should be at levels characteristic of a typical location in a typical
commercial or hospital environment.
NOTE UT is the a.c. mains voltage prior to application of the test level.
MANUFACTURER’S DECLARATION – ELECTROMAGNETIC IMMUNITY
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
-
 65
65
-
 66
66
-
 67
67
-
 68
68
-
 69
69
-
 70
70
-
 71
71
-
 72
72
-
 73
73
-
 74
74
-
 75
75
-
 76
76
-
 77
77
-
 78
78
-
 79
79
-
 80
80
-
 81
81
-
 82
82
-
 83
83
-
 84
84
Therabody 15225120 Mode d'emploi
- Catégorie
- Moniteurs de fréquence cardiaque
- Taper
- Mode d'emploi
dans d''autres langues
Documents connexes
-
 Therabody Vibrationsmassagegerät "Theragun Mini" Manuel utilisateur
Therabody Vibrationsmassagegerät "Theragun Mini" Manuel utilisateur
-
 Therabody Lounger Manuel utilisateur
Therabody Lounger Manuel utilisateur
-
Therabody PROBDL-MP2-PKG-US Mode d'emploi
-
 Therabody Recovery Pulse Manuel utilisateur
Therabody Recovery Pulse Manuel utilisateur
-
Therabody B09QTQDY3B Manuel utilisateur
-
 Therabody RecoveryTherm Manuel utilisateur
Therabody RecoveryTherm Manuel utilisateur
-
Therabody TheraCup Manuel utilisateur
Autres documents
-
Theragun Elite Manuel utilisateur
-
Nuface NuBODY Mode d'emploi
-
 TensCare UNIWAND Manuel utilisateur
TensCare UNIWAND Manuel utilisateur
-
Panasonic EHXT20 Mode d'emploi
-
 Nuface Trinity/PRO Manuel utilisateur
Nuface Trinity/PRO Manuel utilisateur
-
Össur COLD RUSH COMPACT Instructions For Use Manual
-
 Tria POSITIVELY CLEAR Guide de démarrage rapide
Tria POSITIVELY CLEAR Guide de démarrage rapide
-
Enraf-Nonius Sonopuls 492 Manuel utilisateur
-
PROJECT E BEAUTY PE731 Manuel utilisateur
-
Enraf-Nonius Endomed 482u Manuel utilisateur


























































































