
Wireless Pulse Oximeter
Oxymètre de pouls sans l
Ossimetro wireless per il rilevamento del battito
Pulsioxímetro inalámbrico
Funkgesteuertes Pulsoximeter
Oxímetro de Pulso Wireless
Draadloze Pulse-Oxymeter
Ασύρματο Οξύμετρο Παλμού
OPERATION MANUAL
Manuel de presentation
Manuale dell’utente
Manual de Introducción
Bedienungsanleitung
Manual de Funcionamento
Gebruikshandleiding
Εγχειρίδιο Λειτουργίας

MODEL PO3M
Wireless Pulse Oximeter
OPERATION MANUAL
TABLE OF CONTENT
EN
SYMBOLS
INTENDED USE
PARTS AND DISPLAY INDICATORS
PARTS AND DISPLAYS
DEVICE DESCRIPTION
CONTRAINDICATIONS
WARNINGS
Notice
USING YOUR PULSE OXIMETER
CARE AND MAINTENANCE
SPECIFICATIONS
TROUBLESHOOTING
IMPORTANT INFORMATION REQUIRED BY THE FCC
IMPORTANT INFORMATION REQUIRED BY THE INDUSTRY CANADA
Manufacturer Information
ELECTROMAGNETIC COMPATIBILITY INFORMATION
1
2
2
3
3
4
4
4
6
9
10
12
13
13
14
15

SYMBOLS
The symbols below associate with your PO3M
1
Symbols
Symbol for” THE OPERATION MANUAL MUST BE READ”
Symbol for “WARNING”
Symbol for “Type BF Applied Part”
Symbol for ”ENVIRONMENT PROTECTION-Waste electrical products
should not be disposed of with household waste. Please recycle
where facilities exist. Check with your local Authority of retailer for
recycling advice”.
Symbol for “no alarm for SpO2”
Symbol for “Use by date”
Symbol for “Manufacturer”
Symbol for “SERIAL NUMBER”
Symbol for “EUROPEAN REPRESENTATIVE”
Symbol for “KEEP DRY”
Symbol for “COMPILIES WITH MDD93/42/EEC REQUIREMENTS”
Denition of Symbol
SN
EC REP

INTENDED USE
The PO3M Wireless Pulse Oximeter is a non-invasive device intended for spot-checking
of functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate.
The wireless pulse oximeter is intended to measure blood oxygen saturation and pulse
rate of adults above 16 years old in home and hospital environments (including clinical
use in internist/surgery, anesthesia, intensive care, etc.).
The Wireless Pulse oximeter is not intended for continuous monitoring.
Compatibility
The Wireless Pulse Oximeter PO3M is designed for use with the following devices:
iPhone 4S or later
iPad Air or later
iPad mini or later
iPad (3rd generation) or later
iPod touch (5th generation) or later
The iOS version of these devices should be V5.0 or higher.
The Wireless Pulse Oximeter PO3M is also compatible with a number of Android
devices,the Android version should be V4.3 or higher. For a complete list of compatible
devices, please visit the support page on www.ihealthlabs.com
PARTS AND DISPLAY INDICATORS
One (1) Wireless Pulse Oximeter PO3M
One (1) Lanyard
One (1) Operation Manual
One (1) Quick Start Guide
One (1) USB cable
2

DEVICE DESCRIPTION
PARTS AND DISPLAYS
3
Display screen
Bluetooth indicator
Start button
PO3M pulse oximeter measures the amount of oxygen in
your blood and the pulse rate. The oximeter works by
shining two light beams into the small blood vessels or
capillaries of the nger; the measured signal is then
obtained by a photosensitive element and processed by
the microprocessor. The oxygen saturation (SpO2) is
measured as a percentage of full capacity.
Typically, a SpO2 reading between 94%-99% is considered normal. High altitudes and
other factors may aect what is considered normal for a given individual. Concerns
about your readings should be shared with your physician or healthcare professional.

4
IEC 60601-1: 2005 + CORR. 1 (2006) + CORR. 2 (2007)/EN 60601-1:2006 /AC:2010
Medical electrical equipment-Part1: General requirements for basic safety and essential
performance
IEC60601-1-2:2007/EN 60601-1-2:2007 /AC:2010 (Medical electrical equipment -- Part
1-2: General requirements for basic safety and essential performance - Collateral
standard: Electromagnetic compatibility - Requirements and tests)
IEC 60601-1-11 (First Edition): 2010 Medical electrical equipment-Part 1-11: General
requirements for basic safety and essential performance – Collateral Standard:
Requirements for medical electrical equipment and medical electrical systems used in
the home healthcare environment
ISO 80601-2-61:2011 (Medical electrical equipment-Particular requirements for the
basic safety and essential performance of pulse oximeter equipment for medical use).
Hereby, [Andon Health CO.,LTD], declares that this [PO3M] is in compliance with the
essential requirements and other relevant provisions of Directive 1999/5/EC. Directive
1999/5/EC declaration of conformity can be downloaded on the following link :
https://www.ihealthlabs.eu/support/certications
CONTRAINDICATIONS
The PO3M Wireless Pulse Oximeter cannot be used on infant babies.
WARNINGS
1.This device is for use on adults only.
2.Certain activities may pose a risk of injury, including strangulation, if the lanyard
should become wrapped around your neck. Use the lanyard with caution.
3.Do not use the device in a magnetic resonance (MR) environment.
Notice

5
1.Do not use the device as the only basis for making medical decisions. It is intended
only to be used as additional information that you can give to your licensed health
care professional.
2.The device might misinterpret excessive movement as good pulse strength. Limit
nger movement as much as possible when using the device.
3.Do not use the device on the same hand/arm when using a blood pressure cu or
monitor.
4.The device has no alarms of blood oxygen saturation and pulse rate, and it will not
sound if the amount of oxygen in your blood is too low or your pulse rate is abnormal.
If the measurement of SpO2 and pulse rate is not in the normal range, please contact
your health care professional.
5.Do not place the device in liquid or clean it with agents containing ammonium
chloride or products that are not listed in this Operation Manual.
6.Any of the following conditions may reduce the performance of the device:
a)Flickering or very bright light;
b)Excessive Movement;
c)Weak pulse quality (low perfusion);
d)Low hemoglobin;
e)Nail polish, and/or articial nails;
f)Any tests recently performed on you that required an injection of intravascular dyes
7. The device may not work if you have poor circulation. Rub your nger to increase
circulation, or place the device on another nger.
8.The device measures oxygen saturation of functional hemoglobin. High levels of
dysfunctional hemoglobin (caused by sickle cell anemia, carbon monoxide, etc.)
could aect the accuracy of the measurements.
9.Do not use the device in a combustible environment (oxygen enriched environ
ment).

6
10.Do not use the device outside the specied operating temperature range, and do
not store the device outside the specied storage temperature ranges
11.The materials used in the device conform to the biocompatibility and nontoxic
regulations and present no hazard to the body.
12.Use in emergency vehicles with communication systems may aect accuracy of the
measurements.
13.The packaging of the device is recyclable, and it must be collected and disposed
according to the related regulation in the country or region where the package of
the device or its accessories is opened.
14.Any material of the device that may cause pollution must be collected and disposed
according to local rules and requirements.
15.Any single functional tester cannot be used to assess the accuracy of a pulse
oximeter.
16.Do not stare at the lighting LED, as it may irritate your eyes.
17.The device is calibrated to display FUNCTIONAL OXYGEN SATURATION
18.Do not use the device for more than 30 minutes.
19.The wavelength range of pulse oximeter can be especially useful to clinicians
20.Because the pulse oximeter measurements are statistically distributed, only about
two-thirds of pulse oximeter measurements can be expected to fall within ±Arms of
the value measured by a oximeter.
21.The SpO2 accuracy was tested by comparing it to a Co-oximeter and the pulse rate
accuracy was tested by comparing it to a function tester.
22.The device shall not be installed close to or stacked with other devices. When it is
necessary to be close to or stacked with other devices, please observe if the device
can operate normally under such setting rst. For recommended measures of
avoiding or reducing such interference, please refer to the section of "ELECTROMAG-
NETIC COMPATIBILITY INFORMATION".

Charge the battery before rst use
Link the wireless Pulse Oximeter to a USB port of a electrical power source (may be a
PC), and press the “start” button. Then the battery indicator " " will blink, which
means the battery charging is started. When the battery indicator " " stops blinking,
it means the battery has
been fully charged.
DOWNLOAD THE APP ON IOS :
Download the iHealth MyVitals app from Apple app store and install it. (Your
compatible IOS device should be version 5.0 or later)
7
USING YOUR PULSE OXIMETER
Before Using Pulse Oximeter
The wireless pulse oximeter may be used when the user is seated, standing or lying
down. The user should not walk or run during measurements and should take care of
not excessively moving the nger where the device is attached and the corresponding
hand and arm.
It is recommended that the user should wash hands before use. Nail polish, especially
dark shades, may aect the accuracy of the measurement and it is suggested that any
polish be removed prior to monitoring.
The device is suitable for using on any nger excluding the thumb. It is preferred to use
the index or middle nger.

8
DOWNLOAD THE APP ON ANDROID :
Download the iHealth MyVitals app from Google play store and install it. .(Your
compatible Android device should be version 4.3 or later and 4.0 bluetooth).
Create User and Cloud Account
After downloading the app, register and set up your user account following the
on-screen instructions.
When setting up your user account, you will also have access to a free, secure iHealth
Cloud account. Go to www.ihealthlabs.com, then click “Sign In” to access your cloud
account fromPC or Mac.

9
USING WITHOUT SMART DEVICE
1. Clean the device once per week or more frequently if handled by multiple users.
2. Wipe the device with a soft cloth dampened with rubbing alcohol to avoid cross
infection. Do not pour the alcohol directly on or into the device. Dry with a soft
cloth, or allow to air dry.
3. Avoid dropping this device on a hard surface.
4. Do not immerse the device in water or other liquid, as this will result in damage to
the device.
5. If this device is stored below 0 , please acclimate the device to room temperature
before use.
6. Do not try to disassemble this device.
7. The PO3M is a precision electronic instrument and must be repaired/serviced by an
accredited iHealth service center.
8. Incorrect replacement of battery by inadequately trained personnel could result in
an unacceptable risk (e.g., excessive temperatures, re or explosion).
9. The patient simulator « Index 2 », made by the Fluke company, can be used to verify
operation of the oximeter.
10.The expected service life of the PO3M is about 5 years.
CARE AND MAINTENANCE
After it has been used for the rst time, the date and time of the Pulse Oximeter PO3M
will be synchronized with your device. It can also be used without being connected to
an smart device. In this case, the measurement data is stored in the memory and can be
uploaded to the app when the connection is re-established. The Pulse Oximeter PO3M
can store up to 100 measurements. When the memory is full, any new measurement
overwrites the oldest ones.

1.Model: PO3M
2.Classication: Internally powered, type BF
3.Enclosure degree of ingress protection: IPX1
4.Display System: LED
5.Power Source: battery 3.7V Lithium-ion 330mAh
6.Peak wavelength: 660nm/880nm;
7.Maximum optical output power: 1mW;
8.SpO2 Measuring Range: 70-99%
9.Average Root Mean Square (ARMS) of SpO2 Accuracy: 80% 99% ±2%, 70% 79%
±3% ,<70%: no denition.
10.
SPECIFICATIONS
The gure below shows the graphical plot of all SaO2 versus SpO2 with linear regression
t for all the sample data in the clinical protocol.
Range
90%~100%
80%~89%
70%~79%
Arms
1.2215
1.3282
1.7277
10

Scatter plot of SaO2 versus SpO2 with linear
regression t
The gure below shows the graphical plot of
SaO2 versus error (SpO2 – SaO2) with upper
95% and lower 95% limits of agreement:
Scatter plot of dierence between methods
against the SaO2
11.Pulse Rate Measuring Range: 30/min-250/min
12.Pulse Rate Accuracy: 30/min ~ 99/min: ±2, 100/min ~ 250/min: ±2%.
13.Data update period: 15s
14.Automatic Shut-o: After 8 seconds of no indication on the sensors
15.Operation Environment: 5 -40 ; Humidity <80%; Atmospheric pressure:
700hPa-1060hPa
16.Storage Environment: -20 -55 ; Humidity <95%; Atmospheric pressure:
700hPa-1060hPa
11

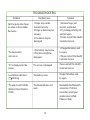
TROUBLESHOOTING
12
Problem Possible Cause
Solution
SpO2 or pulse rate shows
no value, or the number
uctuates.
1.Finger may not be
inserted correctly.
2.Finger or hand may be
moving.
3.The device may be
damaged
1.Remove nger and
reinsert, as directed.
2.Try to keep perfectly still
and test again.
3.Please contact the iHealth
Customer Service
The device does
not turn on.
1.The battery may be low.
2.The device might be
damaged.
1.Charge the battery and
try again.
2.Please contact the iHealth
Customer Service
“E1” is displayed on the
screen
The sensor is damaged
Please contact the iHealth
Customer Service
Low Battery indicator is
blinking.
The battery is low.
Charge the battery and
try again.
The app cannot nd the
Wireless Pulse Oximeter
PO3M.
The Bluetooth does not
work
Reestablish the Bluetooth
connection. If still not
successful, restart your
wireless device (iPod,
iPhone or iPad).

try again.
13
IMPORTANT INFORMATION REQUIRED BY THE FCC
This device complies with Part 15 of the FCC Rules. Its operation is subject to the
following two conditions:
(1) This device may not cause harmful interference.
(2) This device must accept any interference received, including interference that may
cause undesired operation.
Note: This product has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
product generates, uses, and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this product does cause harmful interference to radio or
television reception, which can be determined by turning the equipment of and on, the
user is encouraged to try to correct the interference by one or more of the following
measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit dierent from that to which the
receiver is connected.
—Consult the dealer or an experienced radio/TV technician for help.
IMPORTANT INFORMATION REQUIRED BY THE INDUSTRY CANADA
Under Industry Canada regulations, this radio transmitter may only operate using an
antenna of a type and maximum (or lesser) gain approved for the transmitter by

Industry Canada. To reduce potential radio interference to other users, the antenna
type and its gain should be so chosen that the equivalent isotropically radiated power
(e.i.r.p.) is not more than that necessary for successful communication.
This device complies with Industry Canada license-exempt RSS standard(s). Operation
is subject to the following two conditions: (1) this device may not cause interference,
and (2) this device must accept any interference, including interference that may cause
undesired operation of the device.
14
Manufacturer Information
ANDON HEALTH CO., LTD.
No. 3 Jinping Street, YaAn Road, Nankai District, Tianjin 300190, China.
Tel: 86-22-60526081
IHealthLabs Europe SARL
3 rue Tronchet, 75008, Paris, France
Tel: +33 1 44 94 04 81
Email: [email protected]
Health is a trademark of iHealth Lab Inc. Bluetooth® associated logos are registered
trademarks owned by Bluetooth SIG, Inc. and any use of such marks by iHealth Lab Inc. is
permitted under license. Other trademarks and trade names are those of their respective
owners.
EC REP

ELECTROMAGNETIC COMPATIBILITY INFORMATION
15
Table 1
For all ME EQUIPMENT and ME SYSTEMS
Guidance and manufacture’s declaration - electromagnetic emissions
The PO3M is intended for use in the electromagnetic environment specied below.
The customer or the user of the PO3M should assure that it is used in such an environment.
Emissions test
Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The PO3M uses RF energy only for its internal
function. Therefore, its RF emissions are very
low and are not likely to cause any interference
in nearby electronic equipment.
RF emissions
CISPR 11
Class B
The PO3M is suitable for use in all establish-
ments other than domestic and those directly
connected to the public low-voltage power
supply network that supplies buildings used
for domestic purposes.
Harmonic emissions
IEC/EN 61000-3-2
Not applicable
Voltage uctuations/
icker emissions
IEC/EN 61000-3-3
Not applicable

Table 2
For all ME EQUIPMENT and ME SYSTEMS
16
Guidance and manufacturer’s declaration - electromagnetic immunity
The PO3M is intended for use in the electromagnetic environment specied below. The
customer or the user of the PO3M should assure that it is used in such an environment.
IMMUNITY
test
IEC 60601test
level
Compliance
level
Electromagnetic environment
- guidance
Electrostatic
discharge (ESD)
IEC/EN 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be wood, concrete
or ceramic tile. If oors are covered
with synthetic material, the
relative humidity should be at
least 30 %.
Power frequency
(50/60 Hz)
magnetic eld
IEC/EN 61000-4-8
3 A/m 3 A/m
Power frequency magnetic elds
should be at levels characteristic of
a typical location in a typical
commercial or hospital
environment.
NOTE: UT is the A.C. mains voltage prior to application of the test level.

17
Table 3
For ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Guidance and manufacturer’s declaration - electromagnetic immunity
The PO3M is intended for use in the electromagnetic environment specied below.
The customer or the user of the PO3M should assure that it is used in such an environment.
IMMUNITY
test
IEC/EN 60601
test level
Compliance
level
Electromagnetic environment
- guidance
Radiated RF
IEC/EN 61000-4-3
3 V/m 80 MHz to
2.5 GHz
3 V/m
Portable and mobile RF
communications equipment
should be used no closer to any
part of the [PO3M], including
cables, than the recommended
separation distance calculated from
the equation applicable to the
frequency of the transmitter.
Recommended separation
distance
d=1.2 p
80 MHz to 800 MHz
d=2.3 p
800 MHz to 2,5 GHz
Where P is the maximum output
power rating of the transmitter in
watts (W) according to the
transmitter manufacturer and d is

NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
aected by absorption and reection from structures, objects and people.
A) Field strengths from xed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to xed RF transmitters, an electromagnetic site survey should be
considered. If the measured eld strength in the location in which the PO3M is used exceeds
the applicable RF compliance level above, the PO3M should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating the [PO3M].
B) Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than [V1] V/m.
18
the recommended separation
distance in meters (m).
Field strengths from xed RF
transmitters, as determined by an
electromagnetic site survey,a should
be less than the compliance level in
each frequency range.b
Interference may occur in the vicinity
of equipment marked with the
following symbol:
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
La page charge ...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
-
 53
53
-
 54
54
-
 55
55
-
 56
56
-
 57
57
-
 58
58
-
 59
59
-
 60
60
-
 61
61
-
 62
62
-
 63
63
-
 64
64
-
 65
65
-
 66
66
-
 67
67
-
 68
68
-
 69
69
-
 70
70
-
 71
71
-
 72
72
-
 73
73
-
 74
74
-
 75
75
-
 76
76
-
 77
77
-
 78
78
-
 79
79
-
 80
80
-
 81
81
-
 82
82
-
 83
83
-
 84
84
-
 85
85
-
 86
86
-
 87
87
-
 88
88
-
 89
89
-
 90
90
-
 91
91
-
 92
92
-
 93
93
-
 94
94
-
 95
95
-
 96
96
-
 97
97
-
 98
98
-
 99
99
-
 100
100
-
 101
101
-
 102
102
-
 103
103
-
 104
104
-
 105
105
-
 106
106
-
 107
107
-
 108
108
-
 109
109
-
 110
110
-
 111
111
-
 112
112
iHealth PO3M Manuel utilisateur
- Taper
- Manuel utilisateur
- Ce manuel convient également à
dans d''autres langues
- italiano: iHealth PO3M Manuale utente
- English: iHealth PO3M User manual
- español: iHealth PO3M Manual de usuario
- Deutsch: iHealth PO3M Benutzerhandbuch
- Nederlands: iHealth PO3M Handleiding
- português: iHealth PO3M Manual do usuário
Documents connexes
-
iHealth PO3M Wireless Pulse Oximeter Manuel utilisateur
-
iHealth Air PO3M Guide de démarrage rapide
-
iHealth PO3, PO3M Wireless Pulse Oximeter Manuel utilisateur
-
iHealth Edge AM3S Guide de démarrage rapide
-
iHealth Lite HS4S Guide de démarrage rapide
-
iHealth Lite HS4S Manuel utilisateur
-
iHealth VIEW Manuel utilisateur
-
iHealth Lina HS2 Guide de démarrage rapide
-
iHealth Track KN550-BT Manuel utilisateur
-
iHealth BP3 Guide de démarrage rapide
Autres documents
-
Fora TD-8255B Manuel utilisateur
-
Drive Medical Fingertip Pulse Oximeter Le manuel du propriétaire
-
Drive MQ3000 Le manuel du propriétaire
-
Drive Medical View SpO2 Deluxe Pulse Oximeter Le manuel du propriétaire
-
Drive View SpO2 Deluxe Pulse Oximeter Le manuel du propriétaire
-
Drive Handy-Ox Manuel utilisateur
-
ProfiCare PC-PO 3104 Manuel utilisateur
-
ProfiCare PC-PO 3104 Mode d'emploi
-
Medisana PM 100 Le manuel du propriétaire
-
Mobiclinic Fingertip Manuel utilisateur















































































































