
EM34
Distributed by/Distribuido por/Distribué par:
Beurer North America LP
900 N Federal Highway, Suite 300
Hallandale Beach, FL 33009
www.beurer.com
Questions or comments?
Call toll free 1-800-536-0366 or contact info@beurer.com
¿Preguntas o comentarios? Llame gratis al
1-800-536-0366 o póngase en contacto info@beurer.com
Questions ou commentaires? Appeler gratuitement
1-800-536-0366 ou communiquez avec info@beurer.com
READ THIS MANUAL COMPLETELY AND CAREFULLY BEFORE USING
THIS PRODUCT
Keep this manual in a safe location for future reference
LEA TODO ESTE MANUAL CON ATENCIÓN ANTES DE USAR
ESTE PRODUCTO
Conserve este manual en un lugar seguro para consultarlo en el futuro
LISEZ CE MODE D’EMPLOI COMPLÈTEMENT ET ATTENTIVEMENT AVANT
D’UTILISER CE PRODUIT
Conservez ce manuel en lieu sûr pour y faire référence ultérieurement
ENGLISH
EM34 Knee and Elbow TENS Unit
Instruction manual ....................... 2
ESPAÑOL
TENS para Rodilla y Codo EM34
Manual de instrucciones ........... 15
FRANÇAIS
Unité TENS pour les genoux et les
coudes EM34
Mode d’emploi ........................... 32

2
1. IMPORTANT SAFETY NOTES
Signs and symbols
The following signs appear in the Safety Section (pages 2,
3, 5) and in this manual on pages 6, 10.
READ THIS ENTIRE MANUAL, THE SAFETY SECTION
AND ALL INSTRUCTIONS AND WARNINGS COMPLETE-
LY AND CAREFULLY BEFORE USING THIS PRODUCT.
FOLLOW ALL SAFETY INSTRUCTIONS AND WARNINGS
TO AVOID HAZARDOUS SITUATIONS AND TO MAKE
CORRECT USE OF THIS PRODUCT.
ENGLISH
Contents
1. IMPORTANT SAFETY NOTES ....................................... 2
2. Important information ................................................... 6
3. Parts and Controls ........................................................7
4. Introduction....................................................................8
5. Package Contents ......................................................... 8
6. To Use ............................................................................. 8
7. Care and Maintenance ................................................10
8. Troubleshooting Guide ................................................ 10
9. Frequently Asked Questions ......................................12
10. Specifications ............................................................ 13
12. Warranty ..................................................................... 14
This is the safety alert symbol. It
alerts you to potential personal injury
hazards. Obey all safety messages
that follow this symbol to avoid pos
-
sible injury or death.
WARNING WARNING indicates a hazardous situ-
ation which, if not avoided, could re-
sult in death or serious injury.
CAUTION CAUTION indicates a hazardous situa-
tion which, if not avoided, may result
in minor or moderate injury.
NOTICE NOTICE addresses practices not
related to personal injury, such as
product and/or property damage.

3
WARNING
To reduce the risk of fire, electric shock, or serious
personal injury:
•
This device is for external use on human knees and
elbows only. Do not use this device in the following
situations:
•
If you have implanted electrical devices (e.g. a pace-
maker).
•
If you have metal implants.
•
If you use an insulin pump.
•
In combination with electronic life-sustaining devices,
such as an artificial heart or lung.
•
If you have a fever above 102° F (39° C).
•
If you have cardiac arrhythmia or disorders of the
heart’s impulse and conduction system.
•
After an operation, if muscle contractions could af-
fect the healing process.
•
After consuming alcohol.
•
If you suffer from a seizure disorder (e.g. epilepsy).
•
If you are pregnant.
•
If you have cancer.
•
If you suffer from metabolic arthritis.
•
On acutely or chronically diseased (injured or irritat-
ed) skin (e.g. inflamed skin - whether painful or not,
reddened skin, rashes, e.g. allergies, burns, bruises,
swellings, both open and healing wounds, and post-
operative scars where the healing process could be
affected).
•
On areas of skin that lack sensitivity.
•
In humid environments (e.g. in the bathroom) or when
bathing or showering.
•
In the presence of electronic monitoring systems,
such as heart monitors.
•
While asleep, driving a vehicle, or operating machin-
ery.
•
While using other devices that transmit electrical im-
pulses into your body.
•
Keep the device and accessories away from children.
The device contains small parts that can be swallowed.
•
Store the device and accessories out of the reach of
children. There is a risk of strangulation from the con-
nection cable.
•
This unit is not intended for use by children or persons
with physical, sensory (e.g. insensitivity to pain) or men-
tal impairments or those who do not know how to use it
due to a lack of experience or knowledge, unless they
are supervised by a person responsible for their safe-

4
ty or have received instructions from them on how to
use the unit.
•
The device must not be used in combination with elec-
tronic life-sustaining devices such as ventilators or
other electronic medical products, such as electro
-
cardiographs.
•
If your pain gets worse, becomes chronic, or persists
for up to five days, contact your doctor.
•
Do not share this device with anyone else. This could
lead to skin irritation or infection. The device is intended
for private use only.
•
Do not move or use the cuff if the control unit is
switched on.
•
Only connect the connection cable for the electrode
plug to the intended control unit and the universal cuff
that is included.
•
Do not pull on the connection cables during treatment.
•
Do not bend or pull on the ends of the connection cable.
•
Do not wear any electronic devices, such as watches,
while using the device.
•
During treatment, do not touch the water contact elec-
trodes on the universal cuff with your finger.
•
Do not use the device on a particular area more than
three times a day with a 30-minute treatment period
each time.
•
If the device does not work properly, or you feel unwell
or experience pain, stop using it immediately.
•
Do not modify the electrodes as this is potentially haz-
ardous (max. recommended output value for the elec-
trodes is 58.1 mA/sq in (9 mA/cm²), an effective current
intensity 12.9 mA/sq in (2 mA/cm²) requires increased
awareness).
•
Do not use while undertaking any activity where an un-
expected reaction (e.g. strong muscle contractions even
at low intensity) could be dangerous.
•
Do not use during strenuous activity (e.g. sports).
•
Pre-treating the skin with greasy creams or lotions is
not recommended.
•
At first, use the device while sitting or lying down to
minimize the risk of accidental injuries as a result of
feeling faint. If you feel faint, switch off the device im-
mediately, lie down and support the legs in an elevated
position (approx. 5 - 10 min).
•
Hold the device away from sources of heat and do not
use it within three feet (approx. 1 m) of shortwave or
microwave devices (e.g. mobile phones).
•
The device is intended for self-treatment only.
•
If there are signs of wear and tear or damage or if the
device is used improperly, return it to the manufactur-
er or retailer.

5
•
Do not connect the electrodes to other devices.
•
Do not use in near flammable substances, gases, or
explosives.
•
Do not attempt to open or repair this device; contact
customer service for all repairs.
•
The cuff and the electronic characteristics of this device
could influence the safety and effectiveness of the elec
-
trical stimulation. Do not use any other TENS devices or
cuffs with this product.
•
Before using the device, consult your doctor if any of
the following apply:
•
Serious illness, high blood pressure, a blood coagu-
lation disorder, propensity to thromboembolic condi-
tions or recurrent malignant growths.
•
Skin diseases or open wounds.
•
Unexplained chronic pain in any part of the body.
•
Diabetes.
•
Any sensory impairment that reduces the feeling of
pain (e.g. metabolic disorders).
•
If you are receiving medical treatment.
•
If you are already having stimulation treatment for ex-
isting conditions.
•
If you suffer from persistently irritated skin due to
long-term stimulation at the same electrode site.
NOTICE:
•
D
o not expose the device to direct sunlight or high tem-
peratures.
•
P
rotect the device from dust, dirt and humidity. Never
immerse the device in water or other liquids.
Battery Handling Safety Precautions
• U
se only the size and type of batteries specified.
• B
e sure to follow the correct polarity when installing the
batteries. Reversed batteries may cause damage to the
device.
• D
o not mix different types of batteries together (e.g. Al-
kaline and Carbon-zinc or rechargeable batteries) or old
batteries with fresh ones. Always replace batteries as a
simultaneous set.
• I
f the batteries in the device are depleted or the device
will not be used for a long period of time, remove the
batteries to prevent damage or injury from possible bat-
tery leakage.
• D
o not try to recharge batteries not intended to be re-
charged; they can overheat and rupture (follow battery
manufacturer’s directions).
•
D
o not dispose of batteries in fire, batteries may ex-
plode or leak.

6
• C
lean the battery contacts and also those of the device
prior to battery installation.
•
R
emove discharged batteries from the product and dis-
pose/recycle in compliance with all applicable laws.
•
K
eep batteries away from children and pets. Batteries
may be harmful if swallowed. Should a child or pet swal-
low a battery, seek medical assistance immediately.
2. Important information
Signs and symbols
The following symbols are used in these instructions for
use, on the packaging and on the type plate for the device
and accessories:
Caution
Note
Note on important information
Follow instructions for use
Type BF applied part (cuff only)
Direct current
Keep dry
Pacemaker
S
N
Serial number
Operating conditions
Storage conditions
41 ° F
(5° C)
30 %
75 % 104 ° F
(40° C)
Operating
14 ° F
(-10° C)
10 %
90 %131 ° F
(55° C)
Storage

7
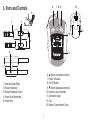
3. Parts and Controls
1
3
2
4
5
1. Hook and Loop Strap
2. Rubber Electrode
3. Rubber Electrode Covers
4. Power Wire Connectors
5. Power Wire
12
13
86
10
11
7 9
6. ▲ Button (increases intensity)
7. Power Wire Jack
8. On/Off Button
9. ▼ Button (decreases intensity)
10. Intensity Level Indicators
11. Illuminated Logo
12. Clip
13. Battery Compartment Cover

8
4. Introduction
The Knee and elbow TENS is a single-channel TENS de-
vice and falls into the category of electrostimulation units.
Gentle electrical pulses are sent via water contact elec-
trodes through the skin to the nerve fibers. The innovative
Knee and elbow TENS provides temporary pain relief in the
knee and elbow area.
TENS, or transcutaneous electrical nerve stimulation, relates
to the electrical stimulation of the nerves via the skin. TENS
is an effective nonpharmacological method of treating dif-
ferent types of pain that have a variety of causes. It has no
side-effects if administered correctly. The method has been
clinically tested and approved and can be used for simple
self-treatment. The pain-relieving or pain-suppressing effect
is achieved by inhibiting the transference of pain to nerve
fibers (caused mainly by high-frequency impulses) and by
increasing the secretion of endorphins in the body. Their ef-
fect on the central nervous system reduces the sensation
of pain. The method is scientifically substantiated and ap-
proved as a form of medical treatment.
Any symptoms that could be relieved using TENS must be
checked by your doctor. Your doctor will also give you in
-
structions on how to carry out a TENS self-treatment re-
gime.
The device is designed to be used for temporary relief for
sore and aching muscles in the upper extremities (arms)
and lower extremities (legs) due to strain from exercise or
normal household and working activities.
5. Package Contents
1 x knee and elbow universal cuff
1 x control unit
1 x connection cable
3 x 1.5 V AAA battery LR03
6. To Use
Battery Installation/Replacement
1
. Remove the Battery Compartment Cover from the bot-
tom of the control unit.
2
. Insert three AAA size alkaline batteries (included), ob-
serving the correct polarity as indicated inside the bat-
tery compartment.
3
. Reattach the Battery Compartment Cover.

9
Connecting Components
1
. Snap the ends of the power wire
onto the power wire connectors on
the cuff. The two ends are inter-
changeable and do not have a
specific left or right.
2
. Moisten the rubber electrode cov-
ers with water, as well as the area
on your body to be treated.
3
. Place the cuff around your knee or
elbow, as desired. Ensure the rub-
ber electrode covers are properly
positioned over the desired treat-
ment area and the opening in the
cuff is over your knee/elbow.
4
. Tighten and secure the hook and
loop straps.
5
. Insert the power wire plug into the
power wire jack on the control unit.
1
. Press the On/Off Button to switch the device on. The
device will beep three times and the logo will flash and
then illuminate.
2
. Use the ▲/▼ buttons to select an intensity level from
1 - 25 that feels most comfortable. The “low” intensity
indicator will flash for levels 1-8, the “middle” intensity
indicator will flash for levels 9-17, and the “high” inten-
sity indicator will flash for levels 18-25. The higher the
level within each range, the faster the indicator will flash.
NOTE: When setting the level, the control unit will beep
once. If it beeps twice, there may not be enough water
on the rubber electrode covers to conduct enough cur-
rent, or the power wire is not attached properly.
3
. This device will switch between two treatment modes by
itself. If you prefer one treatment mode over the other,
you can manually select it by pressing the On/Off Button
during treatment; the Indicator above the “M” will illumin-
ate briefly, indicating that the manual selection mode is
active. To return to auto mode, press the On/Off Button
again; the indicator above the “A” will illuminate briefly.
4
. To shut the device off at any time, press and hold the
On/Off Button until the control unit emits one long beep
and the logo shuts off. It will also shut off by itself after
30 minutes of use.
5
. Always remove the cuff directly after treatment.

10
7. Care and Maintenance
CAUTION
•
Before cleaning, disconnect the power wire from the
device and from the cuff.
Cleaning the Cuff
Clean the cuff in warm soapy water and then rinse well,
making sure all soap is washed out. Pat cuff dry with a towel
and then let air dry completely before use.
Storage
To store the device, first ensure it is switched off. Discon-
nect the power wire from the control unit and from the cuff.
Place all parts back into their original packaging and store
in a cool, dry place out of the reach of children.
8. Troubleshooting Guide
Problem Possible cause Solution
The device
does not
switch on
Batteries are
depleted
Replace the bat
-
teries
Batteries are not
inserted correctly
Reinsert the bat
-
teries according to
the polarity indica-
tors inside the bat-
tery compartment
The elec
-
trical pulses
are weak
Cuff is not con-
tacting skin prop-
erly
Reposition the cuff
to ensure it con
-
tacts the skin suf-
ficiently
Electrodes are not
wet enough
Shut off the de
-
vice and remoisten
electrodes

11
Treatment is
uncomfort
-
able
Power wire is
worn or malfunc-
tioning
Replace the power
wire
Electrodes are
worn or malfunc
-
tioning
Replace the cuff
Intensity level set
too high
Lower intensity
level
Electrodes are not
wet enough
Shut off the de
-
vice and remoisten
electrodes
Irregular
stimulation
Power wire mal
-
functioning
Lower intensity
level and rotate the
power wire plug
90°. If problem
persists, replace
the power wire
Stimulation
is ineffect
-
ive
Electrodes pos-
itioned incorrectly
Remove elec-
trodes and repos-
ition, ensuring cuff
has sufficient skin
contact
The skin
turns red
and/or a
stabbing
pain occurs
The universal cuff
is not positioned
correctly on the
skin
Ensure that the
universal cuff sits
comfortably on the
skin and cannot
move
The universal cuff
is dirty
Clean the universal
cuff as described
in these ins- truc
-
tions for use
One of the water
contact elec
-
trodes has a
scratch
Replace the uni-
versal cuff
Water contact
electrodes are not
sufficiently moist
Switch off the de
-
vice. Moisten elec-
trodes

12
Treatment
stops pre
-
maturely
Cuff has become
too loose
Stop treatment
and retighten/re-
position cuff
Power wire is
loose
Stop treatment
and reinsert power
wire
Batteries are de
-
pleted
Replace batteries
9. Frequently Asked Questions
When should I use this device?
Apply the cuff as soon as you feel pain in your knees/
elbows. If you begin using this device immediately, you
can alleviate or even avoid chronic pain. The earlier you
begin using the device, the higher the probability that pain
can be alleviated.
How often should I use the device?
Do not use the device on a particular area more than three
sessions per day, 30 minutes per session. Start the first
treatment at low intensity until you are familiar with the de-
vice. Stop treatment as soon as the pain has receded or
disappeared. If, after using the device, you experience pain
due to overexertion, stop use for two days. If you experience
further pain due to overexertion, lower the intensity level
during subsequent uses and reduce the treatment time.
When should I stop using the device?
Stop using the device if you feel a stabbing pain during
stimulation. If you notice side-effects following use (skin
irritation, reddening and burning of the skin, headache, feel-
ing unwell), stop using the device. Stop using the device if
pain does not improve after several treatments, becomes
chronic or lasts for more than five days.
What type of pain should I use the device for?
The device works best to treat acute pain, meaning you can
easily determine precisely where the pain is located. Acute
pains are generally located in a specific area of the body and
occur for less than three months. Chronic pain can occur in
different parts of the body and over a period of six months
or more. The device is unable to assist with chronic pain
and you should consult a doctor instead.
This device cannot treat injuries or the source of pain. It is
intended only to provide short-term pain relief with the aim
of improving your day-to-day life.

13
10. Specifications
Output waveform Biphasic rectangular pulse
Pulse length 300 μs
Pulse frequency 2-60Hz or 2/100Hz
Output voltage max. 90 Vpp (500 ohms)
Output current max. 180 mApp (500 ohms)
Power supply 3 x 1.5 V AAA batteries LR03
Intensity Levels 0 to 25
Operating conditions 41 ° – 104 °F (5 °– 40 °C)
at a relative humidity of 30– 75%
Storage conditions 14 °– 131 °F (-10 °– 55 °C)
at a relative humidity of 10– 90%
Dimensions 3.3 x 2.8 x 1.2 in
(85 x 72 x 31 mm)
Weight Control unit: 2.96 oz (84 g)
including batteries
Cuff: 4 oz (113 g)
The serial number can be found on the unit itself or in the bat-
tery compartment.
11. FCC Compliance Statement
This device complies with Part 15 of the FCC Rules. Operation
is subject to the following two conditions: (1) this device
may not cause harmful interference; and (2) this device must ac-
cept any interference received, including interference that may
cause undesirable operation.
NOTE: This equipment has been tested and found to comply
with the limits for a Class B digital device, pursuant to Part 15
of the FCC Rules. These limits are designed to provide reason
-
able protection against harmful interference in a residential in-
stallation. This equipment generates, uses, and can radiate radio
frequency energy, and if not installed and used in accordance
with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interfer
-
ence will not occur in a particular installation. If this equipment
does cause harmful interference to radio or television reception,
which can be determined by turning the equipment off and on,
the user is encouraged to try to correct the interference by one
or more of the following measures:
• R
eorient or relocate the receiving antenna.
•
I
ncrease the separation between the equipment and receiver.
• C
onnect the equipment into an outlet on a circuit different
from that to which the receiver is connected.
•
C
onsult the dealer or an experienced radio/TV technician
for help.
Changes or modifications not expressly approved by the party
responsible for compliance could void the user’s authority to
operate the equipment.

14
12. Warranty
Limited Lifetime Warranty For Original Purchaser
Your Beurer Knee and Elbow TENS Unit, Model EM34, is
warranted to be free from defects in materials and work-
manship for the life of the product under normal condi-
tions of intended use and service. This warranty extends
only to the original retail purchaser and does not extend to
retailers or subsequent owners.
We will, at our option, repair or replace the Beurer Knee
and Elbow TENS Unit, Model EM34, without additional
charge, for any part or parts covered by these written war
-
ranties. No refunds will be given. Repair or replacement
is our only responsibility and your only remedy under this
written warranty. If replacement parts for defective ma
-
terials are not available, Beurer reserves the right to make
product substitutions in lieu of repair or replacement.
For warranty service contact our customer service depart-
ment at 1-800-536-0366 or at info@beurer.com to provide
a description of the problem. If the problem is deemed to
be within the scope of the limited lifetime warranty, you
will be asked to mail the product at your costs in its ori-
ginal package with proof of purchase, your name, address
and phone number. If the problem is not deemed to be
within the scope of the limited lifetime warranty, we will
provide a quotation for repair respectively replacement
and return shipping fee.
This warranty does not cover damage caused by misuse
or abuse; accident; the attachment of unauthorized ac
-
cessory; alteration to the product; improper installation;
misapplication; lack of reasonable care with respect to
the product; unauthorized repairs or modifications; im
-
proper use of electrical/power supply; old worn batteries;
normal wear; loss of power; dropped product; malfunc-
tion or damage of an operating part as a result of failure
to comply with instructions for use or to provide manufac
-
turer’s recommended maintenance; transit damage; theft;
neglect; vandalism; or environmental conditions; loss of
use during the period the product is at a repair facility or
otherwise awaiting parts or repair; or any other condi
-
tions whatsoever that are beyond the control of Beurer.
This warranty is void if the product is ever used in a com-
mercial or business environment. The maximum liability of
Beurer under this warranty is limited to the purchase price
actually paid by the customer for the product covered by
the warranty, as confirmed by proof of purchase, regard
-
less of the amount of any other direct or indirect damage
suffered by the customer.
This warranty is effective only if the product is purchased
and operated in the country in which the product is pur
-
Subject to errors and changes.

15
chased. A product that requires modifications or adapta-
tion to enable it to operate in any other country than the
country for which it was designed, manufactured, ap-
proved and/or authorized, or repair of products damaged
by these modifications is not covered under this warranty.
THE WARRANTY PROVIDED HEREIN SHALL BE THE
SOLE AND EXCLUSIVE WARRANTY. ANY IMPLIED WAR
-
RANTIES, OBLIGATIONS, OR LIABILITES, INCLUDING
BUT NOT LIMITED TO THE IMPLIED WARRANTY OF
MERCHANTABILITY AND FITNESS FOR A PARTICULAR
PURPOSE, ARE LIMITED IN DURATION TO THE DUR
-
ATION OF THIS APPLICABLE WRITTEN WARRANTY.
Some states do not allow limitations on how long an im-
plied warranty lasts, so the above limitations may not
apply to you.
IN NO EVENT SHALL BEURER BE LIABLE FOR ANY
SPECIAL, INCIDENTAL, INDIRECT OR CONSEQUENTIAL
DAMAGES FOR BREACH OF THIS OR ANY OTHER WAR
-
RANTY, EXPRESS, IMPLIED OR ANY OTHER THEORY
OF LIABILITY, WHATSOEVER. Some states do not al-
low the exclusion or limitation of special, incidental, or
consequential damages, so the above limitation may not
apply to you.
Distributed by:
Beurer North America, LP
900 N Federal Hwy, Ste 300
Hallandale Beach, FL 33009 USA
Made in China.

16
1. NOTAS IMPORTANTES DE
SEGURIDAD
Signos ysímbolos
Los siguientes signos aparecen en la sección “Seguridad”
(páginas 16, 19) y en este manual en la página 24.
LEA CON ATENCIÓN TODO ESTE MANUAL, LA SECCIÓN
DE SEGURIDAD YTODAS LAS INSTRUCCIONES YAD-
VERTENCIAS ANTES DE USAR ESTE PRODUCTO. SI-
GA TODAS LAS INSTRUCCIONES YADVERTENCIAS DE
SEGURIDAD PARA EVITAR SITUACIONES PELIGROSAS
YPARA USAR CORRECTAMENTE ESTE PRODUCTO.
ESPAÑOL
Contenido
1. NOTAS IMPORTANTES DE SEGURIDAD ................... 16
2. Información importante .............................................. 20
4. Introducción ................................................................. 22
6. Uso ................................................................................ 23
7. Cuidado y mantenimiento ..........................................25
8. Guía para la resolución de problemas ......................25
9. Preguntas frecuentes .................................................. 27
10. Especificaciones ....................................................... 28
12. Garantía ......................................................................29
Este es el símbolo de adverten-
cia de seguridad. Le pone aler-
ta sobre posibles peligros que
pueden ocasionar lesiones
personales. Tenga en cuenta
en todo momento todos los
mensajes de seguridad des
-
pués de este símbolo para evi-
tar posibles daños o incluso la
muerte.
ADVERTENCIA ADVERTENCIA indica una si-
tuación peligrosa que, si no se
evita, podría causar la muerte
o una lesión grave.

17
PRECAUCIÓN PRECAUCIÓN indica una situa-
ción peligrosa que, si no se evi-
ta, podría causar una lesión me-
nor omoderada.
AVISO AVISO se refiere a prácti-
cas que no están relaciona-
das con lesiones, como es el
caso de daños al producto
o daños materiales.
ADVERTENCIA
Consejos para reducir el riesgo de incendio, descar-
ga eléctrica o lesiones personales graves:
•
Este dispositivo está destinado únicamente para uso
externo en los codos y rodillas de seres humanos. No
utilice este dispositivo en las siguientes situaciones:
•
Si tiene implantado algún dispositivo eléctrico (por
ejemplo, un marcapasos).
•
Si tiene implantes metálicos.
•
Si usa una bomba de insulina.
•
En combinación con dispositivos electrónicos de so-
porte vital, tales como corazones o pulmones arti-
ficiales.
•
Si tiene fiebre alta, por arriba de 39 °C (102 °F).
•
Si tiene una arritmia cardíaca o trastornos del sis-
tema de generación y transmisión de impulsos en
el corazón.
•
Después de una operación, si las contracciones mus-
culares pudieran afectar el proceso de recuperación.
•
Después de ingerir alcohol.
•
Si sufre de algún tipo de trastorno convulsivo (p. ej.
epilepsia).
•
Si está embarazada.
•
Si tiene cáncer.
•
Si sufre artritis metabólica.
•
En piel afectada (lesionada o irritada) de manera agu-
da o crónica: piel inflamada (ya sea dolorosa o no),
piel enrojecida, sarpullidos (por ejemplo, alergias),
quemaduras, moretones, hinchazones, heridas (tan-
to abiertas como en curación) y cicatrices posope-
ratorias en las que pudiera verse afectado el proce-
so de curación).
•
En zonas de la piel con falta de sensibilidad.
•
En ambientes húmedos (p. ej. el baño) o ni en la tina
ni en la regadera.
•
En presencia de sistemas electrónicos de monitoreo,
tales como monitores cardíacos.
•
Mientras duerme, maneja u opera maquinaria.

18
•
Mientras utiliza otros dispositivos que transmitan im-
pulsos eléctricos por su cuerpo.
•
Mantenga el dispositivo y sus accesorios lejos de los
niños. Este dispositivo contiene piezas pequeñas que
son susceptibles de ingerirse.
•
Guarde el dispositivo y sus accesorios fuera del alcan-
ce de los niños. El cable de conexión representa riesgo
de estrangulación.
•
Este dispositivo no debe ser usado por niños o perso-
nas con capacidades físicas, sensoriales (por ejemplo,
insensibilidad al dolor) o mentales restringidas, ni por
personas que carezcan de la experiencia o comprensión
necesarias para el uso del equipo, a menos que estén
bajo la supervisión de una persona que sea responsa
-
ble de su seguridad o hayan recibido instrucciones por
parte de dicha persona sobre cómo usar el dispositivo.
•
Este dispositivo no se debe usar en combinación con
dispositivos electrónicos de soporte vital, tales como
respiradores u otros productos médicos electrónicos
como los electrocardiógrafos.
•
Si el dolor empeora, se vuelve crónico o persiste hasta
cinco días, consulte a su médico.
•
No comparta este dispositivo con nadie más. Esto po-
dría causar irritación o infección de la piel. Este dispo-
sitivo es solo para uso personal.
•
No mueva ni utilice el brazalete si la unidad de control
está encendida.
•
Conecte el cable de conexión para el enchufe de los
electrodos únicamente a la unidad de control y al bra-
zalete universal previstos que se incluyen.
•
No jale los cables de conexión durante el tratamiento.
•
No doble ni jale los extremos del cable de conexión.
•
No utilice dispositivos electrónicos, tales como relojes,
mientras esté usando el dispositivo.
•
Durante el tratamiento, no toque con los dedos los elec-
trodos de contacto con agua que se encuentran en el
brazalete universal.
•
No utilice este dispositivo en una determinada zona más
de tres veces al día, con un periodo de tratamiento de
30 minutos en cada ocasión.
•
Si el dispositivo no funciona correctamente, o si se sien-
te mal o siente dolor, interrumpa su uso de inmediato.
•
No modifique los electrodos, ya que esto es potencial-
mente peligroso (el valor de salida máximo recomenda-
do para los electrodos es de 58.1 mA / sq in (9 mA/cm²);
una intensidad de corriente efectiva mayor de 12.9 mA /
sq in (2 mA/cm²) requiere mayor conciencia).
•
No utilice el dispositivo mientras desarrolla una activi-
dad en la que una reacción inesperada pudiera ser pe-

19
ligrosa (p. ej. las contracciones musculares fuertes pue-
den ser peligrosas incluso a baja intensidad).
•
No utilice el dispositivo durante actividades extenuan-
tes (por ejemplo, el deporte).
•
No se recomienda realizar un tratamiento previo de la
piel con cremas o lociones grasas.
•
Al principio, utilice el dispositivo sentado o acostado
para minimizar el riesgo de lesiones accidentales oca-
sionadas por la sensación de mareo. Si se marea, apa-
gue el dispositivo de inmediato, recuéstese y suba las
piernas (aprox. 5-10 minutos).
•
Mantenga el dispositivo alejado de fuentes de calor y
no lo utilice a menos de tres pies (aprox. 1 m) de dis-
positivos de onda corta o de microondas (por ejemplo,
teléfonos celulares).
•
Este dispositivo es solo para tratamiento personal.
•
Si hay signos de desgaste o daño del dispositivo, o si
este se ha usado en forma indebida, debe regresarse
al fabricante o al distribuidor.
•
No conecte los electrodos a otros dispositivos.
•
No utilice el dispositivo cerca de sustancias inflamables,
gases o explosivos.
•
No intente bajo ninguna circunstancia abrir o reparar el
dispositivo; comuníquese con el servicio al cliente para
realizar cualquier tipo de reparación.
•
El brazalete y las características electrónicas de este
dispositivo pueden influir en la seguridad y efectividad
de la estimulación eléctrica. No utilice ningún otro dis-
positivo TENS ni otros brazaletes con este producto.
•
Antes de utilizar el dispositivo, consulte a su médico
en caso de que sea aplicable a usted alguno de los ca-
sos siguientes:
•
Enfermedades graves, presión arterial alta, trastor-
nos de la coagulación, propensión a enfermedades
tromboembólicas o desarrollos malignos recurrentes.
•
Enfermedades cutáneas o heridas abiertas.
•
Dolor crónico de origen desconocido en cualquier
parte del cuerpo.
•
Diabetes.
•
Cualquier insuficiencia sensorial que reduzca la sen-
sación del dolor (por ejemplo: trastornos metabóli-
cos).
•
Si está recibiendo tratamiento médico.
•
Si ya está bajo tratamiento de estimulación por pro-
blemas previos.
•
Si sufre irritación persistente de la piel a causa de la
estimulación durante un tiempo prolongado con un
electrodo en el mismo sitio.

20
AVISO:
• N
o exponga el dispositivo a la luz directa del sol ni a al-
tas temperaturas.
•
P
roteja el dispositivo contra el polvo, la suciedad y la
humedad. Nunca sumerja el dispositivo en agua ni en
otros líquidos.
Precauciones de seguridad en el manejo
de la batería
•
U
se solo el tamaño y tipo de baterías que se especifican.
•
A
segúrese de utilizar la polaridad correcta cuando insta-
le las baterías. Las baterías colocadas con la polaridad
invertida pueden dañar el dispositivo.
•
N
o combine tipos distintos de baterías (por ejemplo, al-
calinas con carbono-zinc o recargables) ni baterías usa-
das con baterías nuevas. Cuando sustituya las baterías,
sustitúyalas todas al mismo tiempo.
• S
i las baterías del dispositivo están agotadas o el dis-
positivo no se va a utilizar durante un periodo de tiempo
prolongado, retire las baterías para evitar daños o lesio-
nes por un posible derrame de las baterías.
•
N
o intente recargar baterías que no estén diseñadas para
ser recargadas; se pueden sobrecalentar y romper (siga
las instrucciones del fabricante de la batería).
•
N
o arroje las baterías al fuego, ya que podrían explo-
tar o derramarse.
•
L
impie los contactos de las baterías y también los del
dispositivo antes de instalar las baterías.
•
R
etire las baterías descargadas del producto y deseche/
recicle de conformidad con la legislación aplicable.
• M
antenga las baterías lejos del alcance de los niños y
las mascotas. Las baterías pueden ser dañinas en caso
de ingestión. Si un niño o una mascota llegan a inge-
rir una batería, busque atención médica de inmediato.
2. Información importante
Signos y símbolos
Los siguientes símbolos se usan en estas instrucciones
o en el empaque y en la placa de datos del dispositivo y
accesorios.
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
La page est en cours de chargement...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
-
 45
45
-
 46
46
-
 47
47
-
 48
48
-
 49
49
-
 50
50
-
 51
51
-
 52
52
Beurer EM34 Le manuel du propriétaire
- Taper
- Le manuel du propriétaire
- Ce manuel convient également à
dans d''autres langues
- English: Beurer EM34 Owner's manual
- español: Beurer EM34 El manual del propietario
Documents connexes
-
Beurer EM 38 Le manuel du propriétaire
-
Beurer FC 45 Le manuel du propriétaire
-
Beurer IL 50 Le manuel du propriétaire
-
Beurer EM 44 Le manuel du propriétaire
-
Beurer EM26 Manuel utilisateur
-
Beurer EM 27 Le manuel du propriétaire
-
Beurer EM 28 Le manuel du propriétaire
-
Beurer EM28 Manuel utilisateur
-
Beurer EM 27 Manuel utilisateur
-
Beurer BM 55 Le manuel du propriétaire




















































